Question
Question: Draw the cyclic five member ring structure of furan?...
Draw the cyclic five member ring structure of furan?
Solution
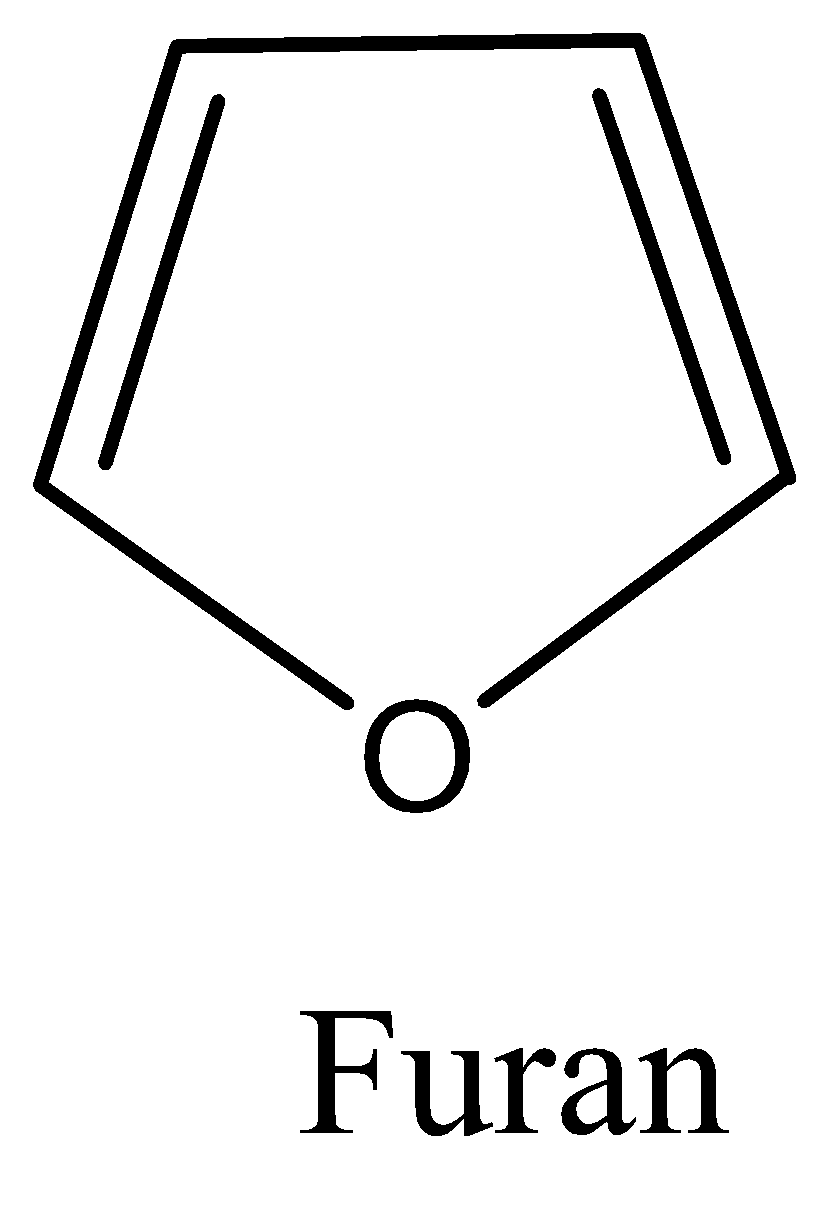
We need to study the structure of furan and produce its ring skeletal structure. Furan is an organic compound which is heterocyclic in nature. A heterocyclic compound is cyclic with atoms of different elements as members of its ring. It has a five-membered aromatic ring with four carbon atoms and one oxygen. It is colourless and highly volatile and compounds containing furan rings are called furans. We now produce the ring structure and explain its structure accordingly.
Complete step by step answer:
We know that furan is a five membered heterocyclic ring which has four carbon atoms and one oxygen atom. The structure is discussed as follows:
Furan is a non-benzenoid hydrocarbon which follows the Huckel’s rule of aromaticity and hence is planar and therefore lacks discrete double bonds.
The oxygen present in the ring has a lone pair of electrons which is sp2 hybridised
The structure is drawn below:
Note: It must be noted that aromatic compounds are of two types which are benzenoids and non-benzenoids. We must have to remember that any hydrocarbon following the Huckel rule can be called aromatic in nature. We must need to remember that the Huckel’s rule states that only planar, fully conjugated monocyclic polyenes having 4n+2π electrons, where n is an integer that is, n=0,1,2,3,4 etc. is aromatic. An aromatic compound must be planar and contain a cyclic cloud of π electrons below and above the plane of the molecule. It contains sp2 hybridized carbon atoms and must obey the Huckel rule.
