Question
Question: Draw the atomic structure of chlorine atom and chloride ion....
Draw the atomic structure of chlorine atom and chloride ion.
Solution
To answer this question, you must recall the arrangement of electrons in the chlorine atom. Chlorine is present in the group 17 of the periodic table and is known as a halogen. The electronic configuration of an element is given by three rules namely, Pauli’s exclusion principle, the Hund’s rule and the Auf- bau principle.
Complete step by step solution
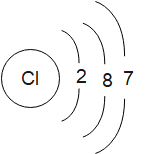
Chlorine is present in the group 17 and the third period of the modern periodic table. It has the atomic number 17. First we must write the electronic configuration of chlorine atoms. The electronic configuration of an element is given by three rules namely, Pauli’s exclusion principle, the Hund’s rule and the Auf- bau principle.
Using the above mentioned rules, we can write the electronic configuration for chlorine atom as:
Cl:1s22s22p63s23p5
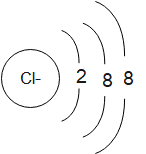
The chloride ion is formed when a chlorine atom accepts an electron in order to complete its octet and attain a stable noble- gas like electronic configuration. The number of protons in the species remains and thus, it carries a single negative charge due to the presence of an extra electron. We can write the electronic configuration of chloride ion as:
Cl−:1s22s22p63s23p6
The structures of the chlorine atom and chloride ion can be drawn as:
Note
The Hund’s rule of maximum multiplicity proposes that all the orbitals in a certain sub- shell are all first singly- filled before the electrons are paired.
Pauli's exclusion principle proposes that each electron in an atom has a different set of quantum numbers that define it.
The Auf- bau principle proposes that the atomic orbitals in an atom are filled in the increasing order of the energy level of the orbitals which is given by the sum of the principal quantum number and Azimuthal quantum number.
