Question
Question: Draw the atomic structure of chlorine atom and chloride ion....
Draw the atomic structure of chlorine atom and chloride ion.
Solution
The atomic structure consists of electrons, protons and neutrons. The proton and neutron are placed in the nucleus and electrons are placed in different orbits around the nucleus. An ion is an electron rich or electron deficient species of the corresponding atom.
Complete step by step answer:
Chlorine is an atom in the periodic table with atomic number 17 and mass number 35. The electronic configuration of chlorine is:
Cl:1s22s22p63s23p5
The valence shell of chlorine atom is 3. It has a total of 17 electrons and 17 protons. The number of neutrons =35−17=18. The 17 protons and 18 neutrons are placed in the nucleus of chlorine. The 17 electrons are placed in different shells of chlorine atoms.
The shells around the nucleus of an atom are labelled asK , L , M , N and so on. The first shell K=1 has two electrons, the second shell K=2 has eight electrons and the third shell M=3 has seven electrons.
Thus the atomic structure of chlorine atoms is shown in figure 1 .
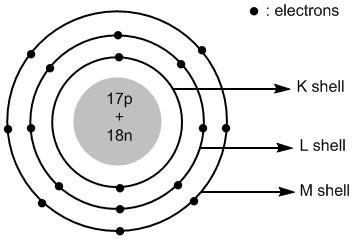
Figure 1 : Atomic structure of chlorine atom.
Chloride ion is an anionic species. It is formed by adding an electron to the chlorine atom. The total number of electrons in chloride ions is 18 . The electronic configuration of chloride ion is:
Cl−:1s22s22p63s23p6
In chloride ion the extra electron is added to the valence shell i.e. third shell or M shell. Thus the atomic structure of chloride ions is shown in figure 2 .
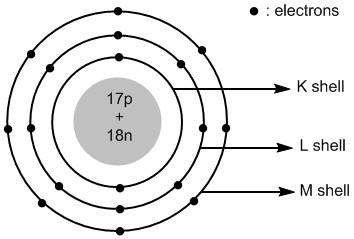
Figure 2 : Atomic structure of chloride ions.
Note:
Like the variance of the number of electrons in chlorine and chloride ions, the number of neutrons also varies. Chlorine exists in two isotopes with mass numbers 35 and37 . Hence the number of neutrons in Cl−35 is 18 while in Cl−37 is 20.
