Question
Question: Draw structure of \(HCl{O_4}\) ....
Draw structure of HClO4 .
Solution
In order to solve the given problem we will study in brief about the given compound and also the different types of bonds present in the structure of the compound further on the basis of the discussion we will draw the structure of the given compound. Also we will see the different characteristics and the uses of the given compound.
Complete step by step answer:
Let us first study in brief about the given compound.
The given compound HClO4 is an acid and its name is Perchloric acid.
HClO4 is an oxoacid containing chlorine with the chemical name Perchloric Acid. Hyperchloric acid HClO4 or hydroxide trioxido chlorine are also identified. A pure odourless colourless aqueous solution is between 50 percent to 72 percent acid. Tissue and metals are corrosive to it. It will rupture violently when closed containers are subjected to heat for a long time.
It can be assembled at a global level in two ways. The high aqueous solubility of sodium perchlorate is thoroughly used in the conventional process. Perchloric acid is formed by the precipitation of solid sodium chloride by treating this solution with hydrochloric acid.
The structure of the Perchloric acid is:
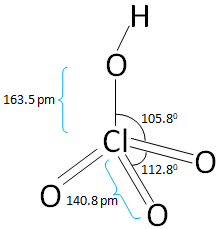
In the above figure of Perchloric acid all the bond length and the bond angles are mentioned.
Now let us see some of the uses of Perchloric acid.
1. Perchloric acid is used in the processing of sodium and potassium as an oxidiser.
2. Perchloric acid used in explosives manufacturing.
3. Perchloric acid used with decorative plating.
4. Perchloric acid used for 1H-Benzotriazole determination as a reagent
5. Perchloric acid utilized as a catalyst.
6. Perchloric acid used in rocket fueling.
7. Perchloric acid used in molybdenum electropolishing.
Note: In order to solve such types of problems related to the diagram of the compound students must remember the different types of bonds that exists between the atoms of different in a compound and should also remember the basic bond length and bond angles for single double bond and different types of geometrical like tetrahedral, octahedral etc.
