Question
Question: Draw structure of \(4 - {\text{methylpent - 1 - yne}}\). How many \(C - H\) sigma, \(C - C\) sigma a...
Draw structure of 4−methylpent - 1 - yne. How many C−H sigma, C−C sigma and C−C pi bonds does it contain?
Solution
Covalent bond is categorized into two parts based on the type of overlapping of orbitals. If the overlapping of orbital is head-to-head overlapping, then the covalent bond formed is known as sigma bond whereas if the overlapping of orbital is sidewise or parallel overlapping, then the covalent bond formed is termed as pi bond.
Complete answer: As per question the compound given is 4−methylpent - 1 - yne. The molecular formula of the given compound is C6H10 and structurally it is represented as follows:
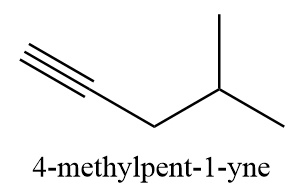
As we know that a single bond consists of only one sigma bond whereas in the formation of a triple bond, the pzorbital approaches the other pz orbital to show sidewise overlapping. Similarly, the other pair of p orbitals i.e., py orbital overlaps sidewise and hence, there are two pi bonds and one sigma bond in a triple covalent bond.
Therefore, in the given compound,
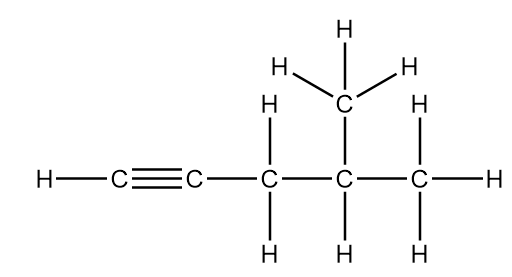
Each hydrogen atom is connected to a carbon atom via a single bond so the number of C−H sigma bonds will be equal to the number of hydrogen atoms present.
So, the number of C−H sigma bond =10
There are a total six carbon atoms in the structure but two of the carbon atoms are not bonded to each other. So, the number of C−C sigma bonds will be less than the number of carbon atoms by one value.
So, the number of C−C sigma bonds =5
Now, there is only one triple bond present in the structure and we already know that there are two pi bonds in a triple bond.
So, the number of C−C pi bonds =2.
Note:
It is important to note that the strength of the bond increases with an increase in the strength of overlapping i.e., greater the overlapping, the stronger is the bond. The extent of overlapping is more in head-to-heat overlapping than sidewise overlapping. So, the sigma bonds are always stronger than pi bonds.
