Question
Question: Draw structure of 2 oxoacids of phosphorus in which oxidation number of phosphorus is \( + 3 \) ....
Draw structure of 2 oxoacids of phosphorus in which oxidation number of phosphorus is +3 .
Solution
An oxoacid (sometimes called an oxyacid) is an oxygen-containing acid. An oxoacid is an acid that contains oxygen, to be more precise. At least one other element is contained. Has at least one oxygen bonded hydrogen atom.
Complete step by step answer
Basically, oxoacids are acids that contain oxygen as an ingredient. As such, a variety of oxoacids are known to form from Phosphorus, such as: H3PO4 , H3PO3 , etc. It is tetrahedrally surrounded by other atoms in phosphorous oxoacids. All these acids are commonly known to form at least one P = O bond and one P-OH bond.
In addition to P= O bonds and P-OH bonds in phosphorus oxoacids where the oxidation state of phosphorus is less than +5 , P-P or P-H bonds are also observed. In general, these acids are seen as too disproportionate to either lower or higher states of oxidation. For instance, it provides phosphine and phosphoric acid when phosphorous acid is heated.
PHOSPHONIC ACID:
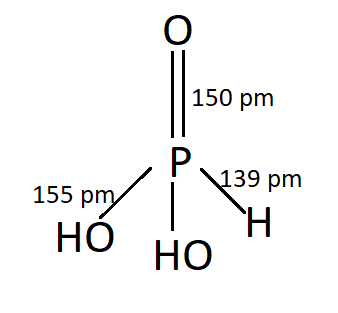
P has an oxidation number of +3 . This is Dibasic.
P4O6+6H2O→4H3PO3
PCl3+3H2O→H3PO3+3HCl
H4P2O5 :
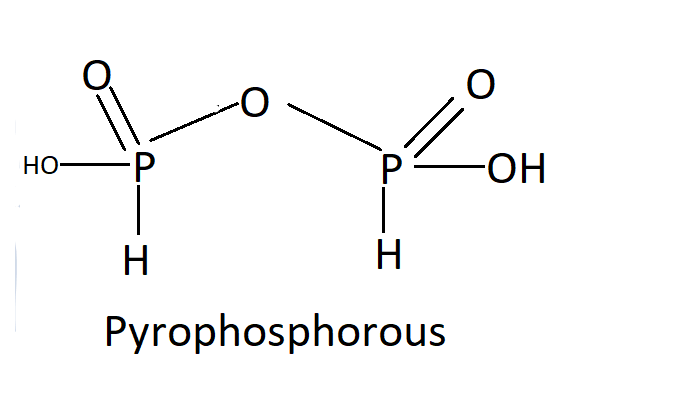
In oxoacids, the P-H bonds cannot go through ionization to provide H+ ions, whereas the H atoms attached to oxygen in P-OH form are ionizable. Therefore, we can assume that only the oxygen-attached H atoms induce basicity. As a consequence of phosphorous acid, H3PO3 is dibasic due to the existence of two P-OH bonds, while H3PO4 is tribasic due to the presence of three P-OH bonds due to phosphoric acid. Phosphorus oxoacids that have P-H bonds have effective reducing properties.
Note
Metaphosphoric acid may not function as a monomer, yet occurs in the form of cyclic metaphosphoric acid as a cyclic trimer or in the form of poly metaphosphoric acid as a linear polymer. Acids with P-H bonds have effective reduction properties.
