Question
Question: Draw structure and write geometry of \(PC{l_3}\) and \(PC{l_5}\)....
Draw structure and write geometry of PCl3 and PCl5.
Solution
VSEPR theory is used to predict the geometry of individual molecules from the number of electron pairs surrounding the central atom. VSEPR Theory is valence shell electron pair repulsion theory.
Complete step by step answer:
As we know VSEPR theory is used to predict the shape of compounds. PCl3 have 3 bond pairs and 1 lone pair. According to this theory if a molecule has 3 bond pairs and 1 lone pair it will possess trigonal pyramidal geometry. The structure of this molecule is shown below:
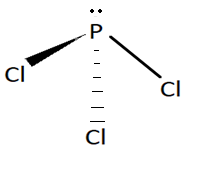
Structure of PCl3
PCl5 has 5 bond pairs and 0 lone pair. According to valence shell electron pair repulsion theory molecules having such bond pair and lone pair possess trigonal bipyramidal geometry. Therefore PCl3 has trigonal bipyramidal geometry. Structure of PCl5 is shown below:
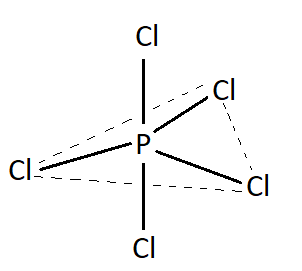
This is the structure ofPCl5.
Additional information:
VSEPR theory was unable to explain the exact shape of molecules in many cases. Taking the direction of electron pairs doesn’t seem to be very rational.
To explain the concept of shapes of molecules clearly hybridization was introduced. It involves intermixing of two or more atomic orbitals of slightly different energies but of the same atom so that redistribution of energy takes place between them resulting in the formation of an equal number of new orbitals which are called hybrid orbitals which will have the same energy, size, and shape.
For molecules or ions having regular geometry, change in electronegativity of the central atom or the surrounding atom has no effect on the bond angle.
The actual structure is in between all the contributing structures and is called a resonance hybrid. The different individual structures are called resonating structures or canonical forms. This phenomenon is called resonance.
Note:
Different compounds have different geometry according to the number of lone pairs and bond pair. So remember the number of bond pairs and lone pairs for which different geometries of compounds are formed.
