Question
Question: Draw R.S and RH ...
Draw R.S and RH
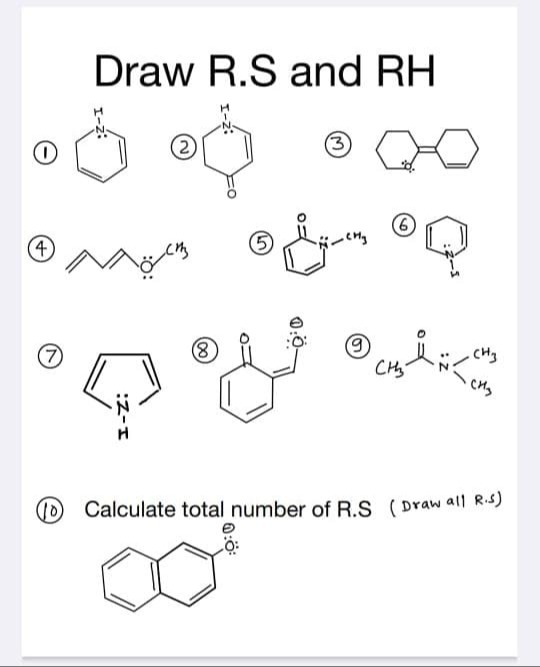
Multiple R.S and RH for each molecule
Solution
Resonance structures (R.S) are different Lewis structures that represent the delocalization of electrons within a molecule or ion. The resonance hybrid (RH) is a weighted average of all significant resonance structures, showing partial bonds and partial charges.
Here's the analysis for each structure:
1. 1,2-Dihydropyridine derivative The molecule contains a nitrogen atom with a lone pair adjacent to a double bond. This allows for conjugation.
- R.S 1 (Original):
H | N: / \ C C | || C C \ / C - R.S 2: The lone pair on N forms a double bond with the adjacent carbon, pushing the pi electrons of the C=C bond onto the carbon at the other end of the double bond, creating a negative charge.
H | N+=C / \ C C- | | C C \ / C - Resonance Hybrid (RH):
The N-C bond has partial double bond character, and the C=C bond also has partial double bond character. The positive charge is partially on N, and the negative charge is partially on the carbon.
H | N(δ+) // \\ C C(δ-) | | C C \\ // C
2. 2-Pyridone This molecule has a nitrogen with a lone pair and a carbonyl group (C=O) conjugated with the ring's double bonds.
- R.S 1 (Original):
O // C
/
C C
|| |
C N-H
\ /
C
```
- R.S 2: Lone pair on N moves to form N=C bond, C=C pi bond moves to adjacent carbon as negative charge.
O // C / \
C C | | C- N+=H \ / C ```
- R.S 3: Negative charge on C forms a double bond, pushing the next C=C pi bond to the adjacent carbon as negative charge.
O // C / \
C C | | C N+=H \ // C- ```
- R.S 4: Negative charge on C forms a double bond, pushing the C=O pi bond onto the oxygen as a negative charge.
O- / C // \
C C | | C N+=H \ // C ```
- Resonance Hybrid (RH):
Partial double bonds exist throughout the ring and for the C-O bond. Partial positive charge on N, partial negative charge on O and carbons C3 and C5 (from the numbering in thought process).
O(δ-) // C // \\ C C || | C(δ-) N(δ+)-H \\ // C(δ-)
3. Cyclohexenyl Radical A radical on an allylic carbon.
- R.S 1 (Original):
. C / \ C C || | C C \ / C - R.S 2: The radical electron pairs with one electron from the adjacent pi bond, and the other electron from the pi bond moves to the other end of the double bond, creating a new radical center.
. C / \ C C | || C C. \ / C - Resonance Hybrid (RH):
The radical character is delocalized over the two carbons. The bonds involved in delocalization have partial double bond character.
. C // \\ C C || | C C. \\ // C
4. Pentadienyl Radical A conjugated radical system.
- R.S 1 (Original):
CH2=CH-CH=CH-CH2. - R.S 2: Radical electron on C5 pairs with C4-C5 pi electron, C3-C4 pi electron moves to C3.
CH2=CH-CH.-CH=CH2 - R.S 3: Radical electron on C3 pairs with C2-C3 pi electron, C1-C2 pi electron moves to C1.
CH2.-CH=CH-CH=CH2 - Resonance Hybrid (RH):
The radical character is delocalized over C1, C3, and C5. All C-C bonds have partial double bond character.
CH2(δ.)--CH=CH--CH=CH2(δ.)
5. N-Methyl-2-pyridone Similar to 2-pyridone, but with a methyl group on N. The resonance structures will be identical in pattern to 2-pyridone, just with -CH3 instead of -H on N.
- R.S 1 (Original):
O // C
/
C C
|| |
C N-CH3
\ /
C
```
- R.S 2: (N+, C5-)
O // C / \
C C | | C- N+=CH3 \ / C ```
- R.S 3: (N+, C3-)
O // C / \
C C | | C N+=CH3 \ // C- ```
- R.S 4: (N+, O-)
O- / C // \
C C | | C N+=CH3 \ // C ```
- Resonance Hybrid (RH):
Similar to 2-pyridone RH, with N(δ+)-CH3.
O(δ-) // C // \\ C C || | C(δ-) N(δ+)-CH3 \\ // C(δ-)
6. Pyridine Aromatic heterocyclic compound. Nitrogen has a lone pair in an sp2 orbital that is not part of the aromatic pi system. The pi electrons are delocalized.
- R.S 1 (Original):
N / \ C C || || C C \ / C - R.S 2: Pi electrons from C=C move towards N, making N negative and an ortho carbon positive.
N- / \ C+ C || || C C \ / C - R.S 3: Positive charge on C propagates through the ring.
N- / \ C C+ || || C C \ / C - R.S 4: Positive charge on C propagates further.
N- / \ C C || || C+ C \ / C - Resonance Hybrid (RH):
Pi electrons are delocalized over all carbons and nitrogen. Nitrogen has a partial negative charge, and carbons at ortho and para positions have partial positive charges.
N(δ-) // \\ C(δ+) C(δ+) || || C C \\ // C(δ+)
7. Pyrrole An aromatic heterocyclic compound. Nitrogen's lone pair is part of the aromatic pi system (contributes 2 electrons to the 6-pi electron system).
- R.S 1 (Original):
N-H / \ C C || || C C \ / C - R.S 2: Lone pair on N moves to form N=C bond, pushing pi electrons of adjacent C=C onto carbon as negative charge.
N+=H // \ C C- || || C C \ / C - R.S 3: Negative charge on C forms double bond, pushing next pi bond to adjacent carbon as negative charge.
N+=H // \ C C || || C- C \ / C - R.S 4: Negative charge on C forms double bond, pushing next pi bond to adjacent carbon as negative charge.
N+=H // \ C C || || C C- \ / C - R.S 5: Negative charge on C forms double bond, pushing next pi bond to adjacent carbon as negative charge.
N+=H // \ C- C || || C C \ / C - Resonance Hybrid (RH):
Nitrogen has a partial positive charge, and all carbons have partial negative charges. Bonds have partial double bond character.
N(δ+)-H // \\ C(δ-) C(δ-) || || C(δ-) C(δ-) \\ // C(δ-)
8. Ortho-hydroxybenzaldehyde anion (phenoxide-like) This is an enolate-type system conjugated with a benzene ring. The structure shows a benzene ring with a C=O group and an adjacent C-O- group (enolate).
- R.S 1 (Original):
O- / C=O / \ C C || || C C \ / C - R.S 2: Negative charge on O forms C=O, pushing C=O pi electrons to adjacent carbon of ring.
O // C-O- / \ C C || || C C \ / C - R.S 3: Negative charge on carbon forms double bond, pushing pi electrons to next carbon.
O // C-O- / \ C C || || C- C \ / C - R.S 4: Negative charge on carbon forms double bond, pushing pi electrons to next carbon.
O // C-O- / \ C C || || C C- \ / C - R.S 5: Negative charge on carbon forms double bond, pushing pi electrons to next carbon.
O // C-O- / \ C- C || || C C \ / C - Resonance Hybrid (RH):
Negative charge is delocalized over the two oxygen atoms and ortho/para carbons of the benzene ring. All C-C bonds in the ring have partial double bond character.
O(δ-) // C(δ-)O(δ-) // \\ C(δ-) C(δ-) || || C(δ-) C(δ-) \\ // C(δ-)
9. N,N-Dimethylacetamide (Amide) The lone pair on nitrogen is conjugated with the carbonyl group.
- R.S 1 (Original):
O // CH3-C \ N(CH3)2 - R.S 2: Lone pair on N forms a double bond with C, pushing C=O pi electrons onto O as negative charge.
O- | CH3-C=N+(CH3)2 - Resonance Hybrid (RH):
The C-N bond has partial double bond character, and the C-O bond has partial double bond character. Oxygen has a partial negative charge, and nitrogen has a partial positive charge.
O(δ-) | CH3-C=N(δ+)(CH3)2
10. Naphthoxide Anion The question asks to calculate the total number of R.S and draw all of them for the naphthoxide anion. The negative charge on the oxygen can delocalize into the naphthalene ring system.
- R.S 1 (Original):
O- / C
//
C C
|| ||
C C
\ /
C
```
- R.S 2: Negative charge on O forms C=O, pushing pi electrons to adjacent carbon (ortho position).
O // C-
//
C C
|| ||
C C
\ /
C
```
- R.S 3: Negative charge on carbon forms double bond, pushing pi electrons to next carbon (para position in the first ring).
O // C
//
C C-
|| ||
C C
\ /
C
```
- R.S 4: Negative charge on carbon forms double bond, pushing pi electrons to carbon at the ring junction.
O // C
//
C C
|| ||
C- C
\ /
C
```
- R.S 5: Negative charge on ring junction carbon forms double bond, pushing pi electrons into the second ring.
O // C
//
C C
|| ||
C C
\ /
C-
```
- R.S 6: Negative charge on carbon in second ring forms double bond, pushing pi electrons to next carbon.
O // C
//
C C
|| ||
C C-
\ /
C
```
- R.S 7: Negative charge on carbon in second ring forms double bond, pushing pi electrons to next carbon.
O // C
//
C C
|| ||
C- C
\ /
C
```
- R.S 8: Negative charge on carbon at ring junction forms double bond, pushing pi electrons back to the first ring.
O // C
//
C C-
|| ||
C C
\ /
C
```
- R.S 9: Negative charge on carbon forms double bond, pushing pi electrons back to oxygen.
O- / C
//
C C
|| ||
C C
\ /
C
```
Total number of R.S = 9.
- Resonance Hybrid (RH):
The negative charge is delocalized over the oxygen atom and several carbons in both rings, particularly at ortho and para positions relative to the oxygen. All C-C bonds have partial double bond character.
O(δ-) // C(δ-)
// \ C(δ-) C(δ-) || || C(δ-) C(δ-) \ // C(δ-) ```
The final answer is Multiple R.S and RH for each molecule
