Question
Question: Draw resonance structures for each of the following compounds. A.
B.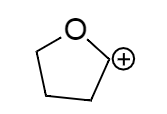
C.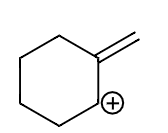
Solution
Resonating structures represent the movement of electron clouds inside a molecule, when it has alternating double bonds.
The more resonating structures a molecule or ion has, the more stable it becomes.
Complete step by step answer:
Resonance is an exercise within a molecule following the Valence Bond Theory of bonding that defines the delocalization of electron clouds within the molecules or ions. It involves constructing several Lewis structures which, when combined together, represents the full electronic arrangement of the molecule. Resonating structures are used when a single Lewis-dot structure cannot fully express the bonding. The combination of all the possible resonating structures is termed as a resonance hybrid, which signifies the overall delocalization of electron clouds within the molecule. generally, the molecule which has multiple resonance structures is supposed to be more stable than one which has fewer, also, some resonance structures have more contribution towards the stability of the molecule than others.
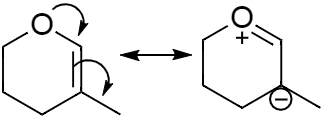
Above shown is the resonating structure of the molecule (a) which was given in the question. This molecule contains only one double bond, so the lone pairs of electrons present in the oxygen will delocalise and in turn the electron cloud of the double bond will shift towards the adjacent carbon.
The resonating structure of the ion (b) is shown below, in this we can see there is a positive charge present in the molecule. So the lone pair of electrons will shift to fill that positive charge, resulting in formation of a resonating structure.
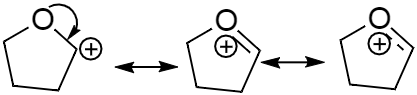
In case of the third molecule (c) the pi electron cloud will shift in order to fill the positive charge and make the ion into a more stable molecule. In the second structure as we can see the double bond is now a part of the ring.
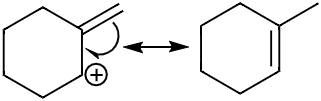
Note: Resonance is a theoretical concept which helps us determine the possible sites where the electrons get localised.
The knowledge of resonating structure can be applied to predict the sites where nucleophilic and electrophilic substituents would get attached.
