Question
Question: Draw orbit structure diagram of Sodium chloride \(\left( {NaCl} \right)\)....
Draw orbit structure diagram of Sodium chloride (NaCl).
Solution
Sodium chloride which is also known as common salt, is an ionic compound whose chemical formula is NaCl. It contains 1:1 ratio of sodium ion and chloride ion. The molar masses of sodium 22.99and 35.45gmol−1respectively.
Complete step by step answer:
The reason for the salinity of seawater and of the extracellular fluid of many multicellular organisms is Sodium chloride. It is commonly used in condiments and food preservatives. Large quantities of sodium chloride are used in industrial processes. It is the main source of sodium and chlorine compounds for feedstocks for further chemical synthesis. The second most important use of sodium chloride is in deicing of roadways in sub-freezing weather.
Preparation of Sodium Chloride:
Chlor-alkali industry: In the industrial process NaCl is used to produce chlorine and sodium hydroxide as shown in the given equation:
2NaCl+2H2O→Cl2+H2+2NaOH
This electrolysis is regulated in either a mercury cell, diaphragm cell or a membrane cell. Each of them is used in a different method to separate the chlorine from the sodium hydroxide.
Soda-ash industry: In the Solvay process, sodium chloride is used to produce sodium carbonate and calcium chloride. For the production of glass, sodium bicarbonate and dyes as well as a myriad of other chemicals, Sodium carbonate is used.
Properties:
Sodium chloride can easily be soluble in water and partially soluble or insoluble in other liquids.
They are white crystals which do not possess an odour but have a taste.
In its aqueous state NaCl behaves as a good conductor of electricity due to the free movement of the ions.
Its melting point is 801∘C and boiling point is 1,413∘C.
The orbit structure of NaCl is:
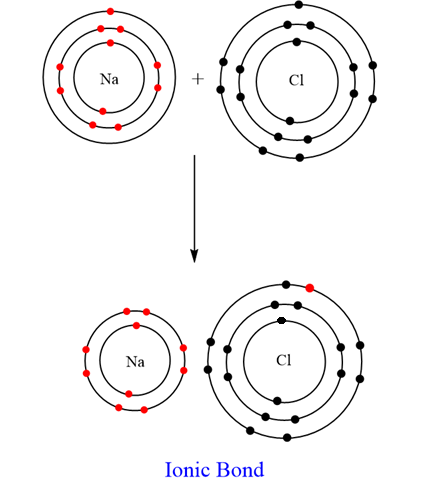
Note:
Sodium chloride is the basic compound used by our body to digest and transport nutrients also termed as salt. It is used for preservation of blood pressure and keeps the correct fluid balance. When a sodium atom interacts with a chlorine atom then sodium chloride is formed.
