Question
Question: Draw electron dot representation for the formation of potassium chloride....
Draw electron dot representation for the formation of potassium chloride.
Solution
As we know that electron dot structure is the diagram that tells us about the chemical bonding between atoms and also the number of lone pairs around them. Since potassium is a metal and chlorine is non-metal, they bond together by transfer of electrons which leads to completion of their octet and hence become a stable compound.
Complete step by step answer: The electron dot structure is the representation of valence electrons around the symbol of an atom. It helps us to understand the chemical bonding of various salt compounds and complexes.
-Potassium: It is an element of s-block with atomic number 19. Its electronic configuration is1s22s22p63s23p64s1. From the electronic configuration we can observe that potassium has 1 electron lying in the outermost shell. Therefore, it can be represented as:-
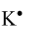
-Chlorine: It is an element of p-block with atomic number 17. Its electronic configuration is1s22s22p63s23p5. From the electronic configuration we can observe that chlorine has 7 electrons lying in the outermost shell. Therefore, it can be represented as:-
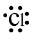
-Formation of Potassium Chloride:-
Potassium chloride is an ionic compound formed by ionic interaction between cation and anion. Since potassium is a metal, it has a tendency to donate electrons and become a cation. When it loses 1 electron, it achieves a stable noble gas configuration. On the other side, chlorine being a non-metal has a tendency to accept electrons and become an anion.
When potassium donates its electron, chlorine easily accepts it and both the ions interact with each other hence resulting in the formation of potassium chloride. The electron dot representation for the formation of potassium chloride is shown below:-

Note: Generally electron dot representation is used to explain chemical bonding of various molecules and compounds but it has majorly 3 exceptions:-
- Molecules which have odd number of electrons.
- Molecules in which one or more atoms consist of more than eight electrons.
- Molecules in which one or more atoms have less than eight electrons.
