Question
Question: Draw electron dot representation for the formation of Hcl....
Draw electron dot representation for the formation of Hcl.
Solution
Hydrogen chloride is a covalently bonded molecule which generally exists as a gas. When added in water it ionizes forming a proton and chloride ion. You must recall the structure of hydrogen chloride in order to answer this question.
Complete step by step solution:
Electron dot structures are also known as lewis dot structures or Lewis structures. They represent the valence electrons of the atoms that constitute a molecule. They help us to visualize the valence electrons of atoms and molecules and the bond formation between them.
IN the electron dot structure, we write the chemical symbol for the element and around it we draw its valence electrons which are represented by dots, hence giving the name as electron dot structure. The number of electrons placed on the molecule must correspond to the number of total valence electrons present in the atom in the molecule.
While drawing the electron dot structures, we need to know that elements form compounds with other elements in order to complete their octet and attain more stability.
Hydrogen has one electron in its valence shell and needs one more to completely fill its 1 s- orbital and attain a noble gas like configuration. Similar is the case of chlorine. It has a total of seven valence electrons and is one electron short of completing its octet and attaining a stable noble gas like configuration. Thus, both the hydrogen and chlorine atoms share an electron with each other forming a covalent bond.
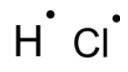
Note:
Hydrochloric acid is a well-known strong acid, that is, it releases a proton. But it happens in aqueous medium. Outside an aqueous medium, hydrogen chloride exists as a covalently bonded gas.
