Question
Question: Draw canonical form of the following: 
Solution
We can say that another name of canonical structure is resonance structure, i.e. one of possibly more than one contributing structures which combine to form the true, resonance hybrid structure. We can say resonance is a way of explaining bonding in some molecules (or) ions by combining many contributing structures into a resonance hybrid.
Complete step by step answer:
We have to know that canonical structure is a way of indicating (or) explaining the delocalized electrons inside several molecules (or) polyatomic ions such as sulfate, phosphate ions where the bonding could not be represented based on Lewis structures. Resonance is the delocalization of pi electrons that are presented in several intermediate structures known as canonical forms.
The resonance hybrid represents the actual molecule as the average of contributing structures with partial charges and bond length taking on intermediate values compared to those for the individual Lewis structure of the contributors where they are found as real chemical entities.
Contributing structures are separated by double headed arrows. In a hybrid structure, pi bonds are involved in resonance.
There are four resonance forms of benzyl carbocation C6H5−CH2+. We can draw the canonical forms of benzyl carbocation as,
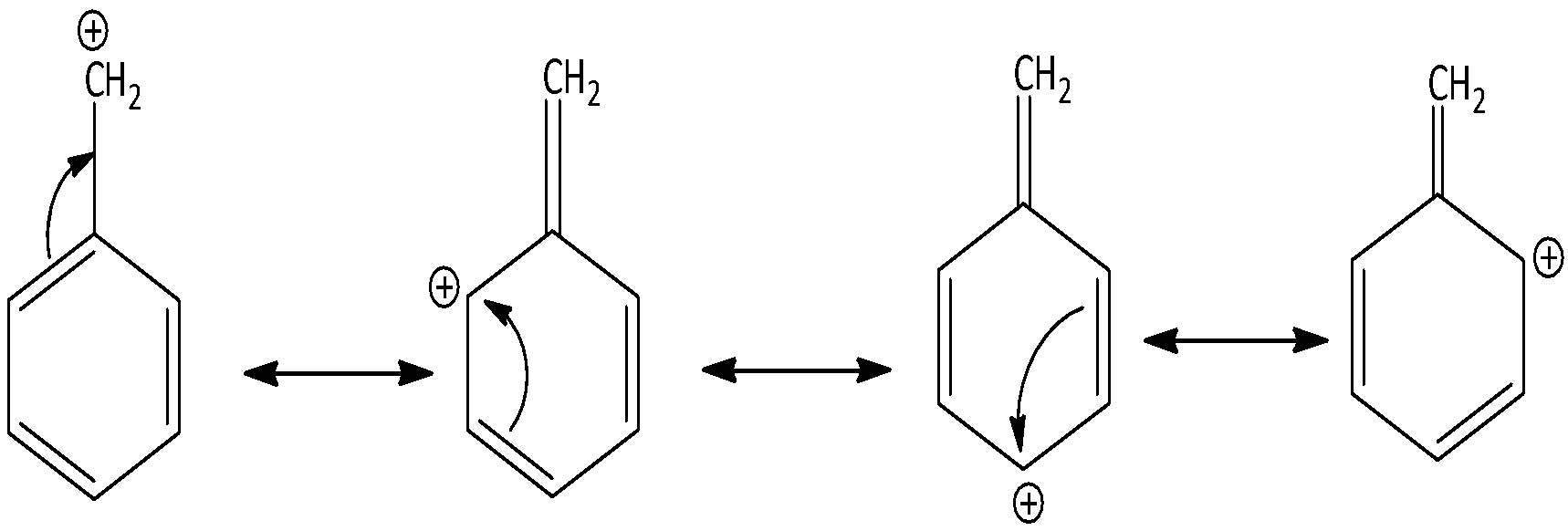
Molecules that have extended pi systems like linear polyenes and polyaromatic compounds are explained by both resonance hydrides and delocalized orbitals in molecular orbital theory.
Note: In other aromatic rings and benzene, a solid circle is used to indicate the delocalized pi electrons. We have to know that resonances and isomerism are two different concepts. We know that isomers are molecules that have the same chemical formula but different in spatial arrangements of atoms. Resonance differs in the way electrons are formally assigned to atoms in Lewis structure descriptions of the molecule.
