Question
Question: Draw \(B{{F}_{3}}\) and assign a point group. How many degrees of vibration freedom does the molecu...
Draw BF3 and assign a point group. How many degrees of vibration freedom does the molecule have?
Solution
Degree of freedom is the quantity of variable needed to describe the movement of a molecule completely. For a molecule moving in 3-dimensional space, three coordinates are sufficient so its degree of freedom is three. The Lewis structure of BF3 has 7×3 valence electrons from the fluorine valence electron from the boron.
Complete step by step answer:
Boron trifluoride is the inorganic compound with the formula. BF3 This pungent colourless poisonous fuel emits white fumes in moist air. It is a useful Lewis acid and a flexible constructing block for other boron compounds. A molecule which has 3 bonded atoms and no lone pair of electrons. Boron donates three electrons. with 24 valence electrons, we assign 3×6nonbonding electrons to the fluorines and the closing 6 as three single bonds.
The geometry of the molecule is called trigonal planar. The molecule has no dipole moment via a distinctive feature of its high symmetry.
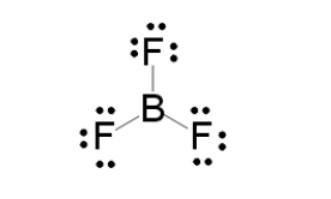
This chemical compound is an inorganic compound that is colourless however poisonous in nature when it's far inside the gaseous level. It reacts with moist air. In its liquid form, its miles are quite soluble (dehydrate) substances.
It's far sp2 hybridized. To provide an explanation for it in simple phrases, Boron’s atomic p orbital and s orbital within the outer shell generally integrate to form 3sp2 hybrid orbitals which are all the equivalent energy.
VIBRATIONAL DEGREES OF FREEDOM:
The degrees of vibrational freedom for nonlinear polyatomic molecules are found as 3N−6
Where N is the number of atoms.
Thus,BF3 has 6 vibrational modes.
Note: All the bonds in BF3 are sigma bonds. Anhydrous boron trifluoride has a boiling factor of −100.3∘C and a critical temperature of so that it may be saved as a refrigerated liquid most effective among the ones temperatures of −12.3∘C. Storage or shipping vessels have to be designed to resist internal pressure. Boron trifluoride is corrosive. It is also used in touchy neutron detectors in ionization chambers and gadgets to screen radiation stages in the Earth's surroundings
