Question
Question: Draw an electron dot diagram to show the formation of ammonium ions....
Draw an electron dot diagram to show the formation of ammonium ions.
Solution
We know that the Ammonium ion consists of nitrogen and hydrogen atoms. It has one nitrogen atom and four hydrogen atoms. Electron dot diagrams can show how the electrons are involved in a bond formation. It is a diagram in which valence electrons of an atom are shown as dots and how they are distributed around the element’s symbol.
Complete step by step solution:
Ammonia consists of one nitrogen and three hydrogen. Nitrogen has five valence electrons. Out of which three electrons of nitrogen form a covalent bond with hydrogen, with sharing of one electron of nitrogen and one electron of hydrogen. There are three such bonds. So, two electrons are left as a lone pair. This is the structure of ammonia (NH3).
In ammonium ions, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is given as follow as:
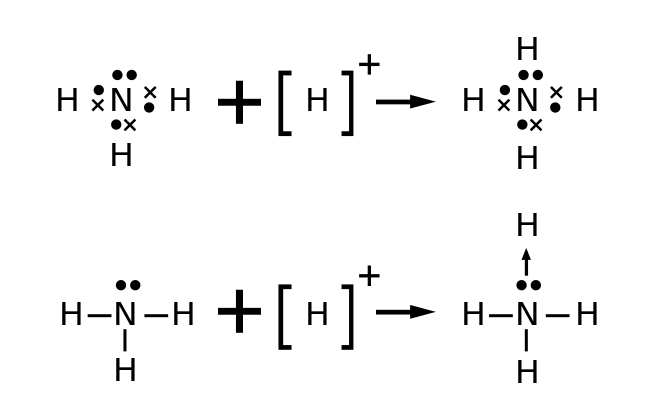
Note:
Remember that both ammonia and ammonium have tetrahedral structure. When ammonia reacts with water ammonium ion is formed by taking up a proton from water. Ammonium ion is the one which can form nontoxic salts. Under normal conditions, both ammonia and ammonium ions will be present in normal water. Usually the dot diagrams are the same for each element in their representative element groups.
