Question
Question: Draw a plot of \[{\text{lo}}{{\text{g}}_{{\text{10}}}}\left[ {\text{R}} \right]\] versus time 't' fo...
Draw a plot of log10[R] versus time 't' for a first order reaction. What is the slope of the line equal to?
Solution
The plot of log10[R] versus time 't' for a first order reaction is a straight line having negative slope. Write the integrated rate law for the first order reaction as log10[R] = (−2.303k)t + log10[R]o. Then compare it to the equation of the straight line Y=mX+C .Here, ‘m’ represents the slope.
Complete Step by step answer: Consider the following first order reaction:
R→P
Here, R is the reactant and P is the product.
The rate of the first order reaction is given by the formula
−dtd[R]=dtd[P]=k[R]
Here, −dtd[R] represents the rate at which the reactant R is consumed. It also represents the rate of the reaction. dtd[P] represents the rate of formation of the product P. k is the rate constant or the specific reaction rate. [R] represents the concentration of the reactant R.
Write down the integrated rate law for the first order reaction.
log10[R] = log10[R]o−(2.303k)t
Rearrange the above rate law expression
log10[R] = (−2.303k)t + log10[R]o
Compare this to the equation of straight line of the type Y=mX+C
Here,
Here, m is the slope and C is the Y-intercept.
When you draw a plot of logio[R] versus time 't' for a first order reaction, a straight line with negative slope is obtained.
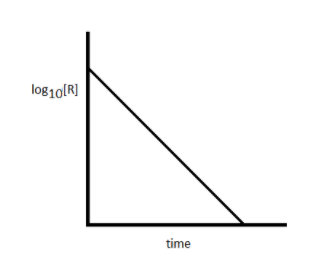
**The slope of line equal to −2.303k
The Y-intercept is equal to log10[R]o **
Note: For the first order reaction, the rate of the reaction is directly proportional to the reactant concentration. If you plot log10[R] versus time 't' for the reaction and obtain a straight line with negative slope, then you can say that the reaction is of first order in nature.
