Question
Question: Draw a neat diagram showing the Permutit process of softening hard water and label the following: (a...
Draw a neat diagram showing the Permutit process of softening hard water and label the following: (a) Zeolite layer (b) Soft water layer.
Solution
Hint: Try to identify the Zeolite layer in which zeolite is regenerated by passing a brine solution. The soft water layer will be above this zeolite layer which is free from impurities. Calcium and Magnesium zeolites settle at the bottom.
Complete step by step answer:
We know that in the Permutit process salts which cause hardness of water are replaced by salts of sodium. Zeolites are made of aluminates and silicates which can attract many cations. In the Permutit process, we use sodium zeolites for softening hard water. The formula of sodium zeolite is Na2Al2Si3O10.2H2O.
This sodium zeolite reacts with salts of magnesium and calcium and converts them to magnesium and calcium zeolites.
Na2Pm+CaSO4⟶CaPm+Na2SO4
Na2Pm+Ca(HCO3)2⟶CaPm+2NaHCO3
Here Pm stands for Permutit or zeolite. Similar reactions occur with magnesium salts.
After sometime sodium zeolite is completely converted to calcium and magnesium zeolites so reactions stop. Then, we pass a 10% brine solution through these calcium and magnesium salts to regenerate back sodium Permutit. And we can reuse this Permutit in softening hard water.
CaPm(orMgPm)+2NaCl⟶Na2Pm+CaCl2(orMgCl2)
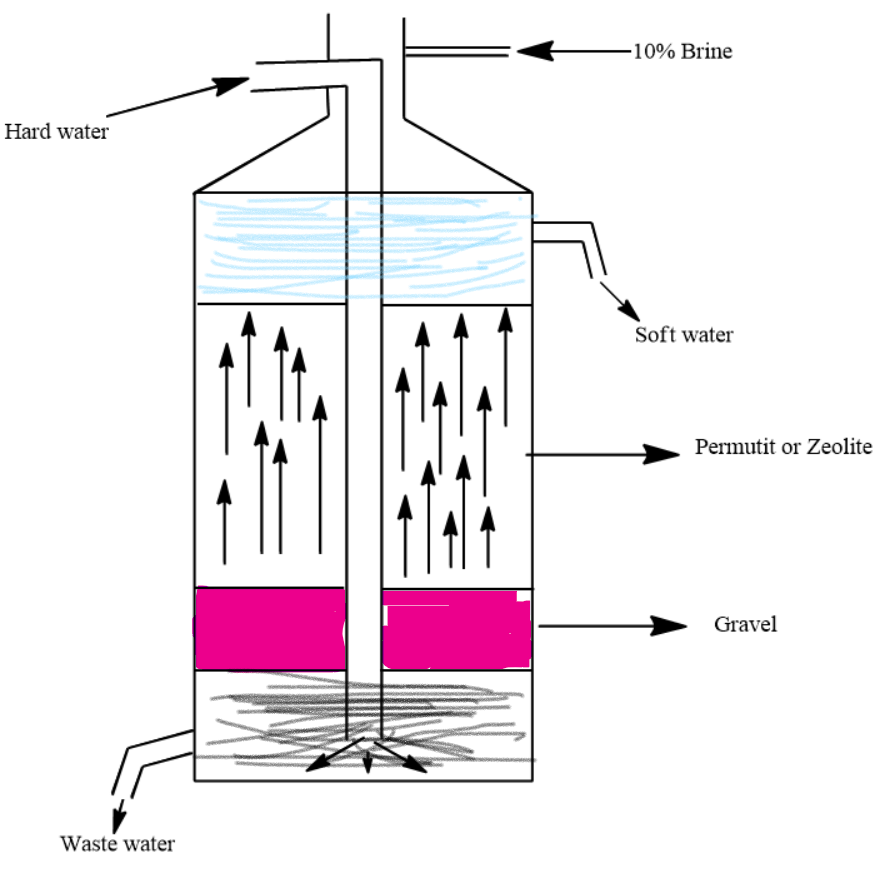
The zeolite layer is below the soft water layer.
Note: If there are some transition metal impurities they need to be removed first. In this reaction as there is no precipitate formation, hard water has no obstruction to come into the chamber. It is a fast process and can be easily managed.
