Question
Question: Double bond equivalent (degree of unsaturation) of (A) is:  of (A) is:
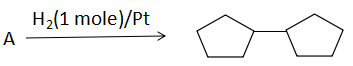
A. 1
B. 2
C. 3
D .4
Solution
Degree of unsaturation can be simply calculated as sum of no. of double bond and no. of ring present in the structure. In this question we have H2/Pt as a catalyst and this catalyst adds two hydrogen atoms to the alkene.
Complete step by step answer:
In this question we have been given a chemical equation in which hydrogenation (addition of hydrogen) occurs by using the catalyst H2/Pt and a product is formed, which has two cyclopentane rings in it and we have been asked what is the unknown reactant. As we know H2 with Pt act on alkyne to give alkene and alkane by adding two H atoms at the double and triple bond centre. One mole of H2/Pt requires for hydrogenation of one bond. In this equation it is given that we have used only 1 mole of H2/Pt . So, the reactant contained only one extra bond as compare to the product. Now we have asked the degree of unsaturation of the reactant.
So, as we know, the degree of unsaturation = no. of double bond + no. of ring present in the structure.
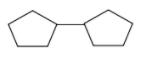
As we can see there are two rings in the product with no double bond. So, the reactant will have 2 ring and one double bond. So, as per our formula no. of ring + no. of double bond, the degree of unsaturation of the reactant will be 2+1=3 .
Therefore, the correct option is option C.
Note:
We can also solve this question by Double bond equivalent =C+1−2H−2X+2N
Here X and N is 0 and C=10 and H=2 less than the product=18−2=16
Hence, DBE=10+1−216=10+1−8=3 S
