Question
Question: Does VSEPR theory predict that \(Xe{F_2}\) is linear?...
Does VSEPR theory predict that XeF2 is linear?
Solution
The valence shell electron pair repulsion theory i.e., VSEPR theory is generally used to predict the molecular geometry of a compound. The basic feature of this theory is that repulsions among the pair of electrons on the central atom, be it a bonding or nonbonding electron pair, will control the overall geometry of the molecule.
Complete answer:
According to VSEPR theory, to decide the geometry of a molecule following steps must be followed:
1. The least electronegative atom is always considered as the central atom since this atom has the highest tendency to share its electrons with other atoms bonded to it.
2. The total number of valence electrons for the central atom must be counted.
3. Add one electron for each bonding atom i.e., count the number of bonds formed by the central atom.
4. Add or subtract the electrons for the charge (if any) for the molecule.
5. Divide the total of above values by 2 to find the total number of electron pairs which is also known as VESP number.
Now, as per above steps the geometry of XeF2 will be as follows:
| Central atom | Xe |
|---|---|
| Valence electrons on central atom | 8 |
| Contributing 1 electron for each fluorine atom | 2 |
| Total | 10 |
| Dividing by 2 to get VESP number | 5 |
| Hybridization | sp3d |
| Number of lone pairs on central atom | 3 |
| Geometry corresponding to VESP number | Linear |
In the XeF2 molecule, there are three lone pairs which occupy the equatorial positions and fluorine atoms occupy axial positions in order to minimize repulsion between lone pair of electrons. The structure of XeF2 can be represented as follows:
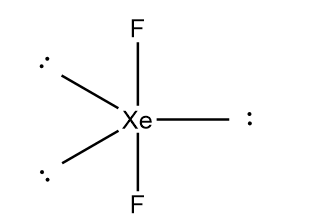
Thus, we can conclude that VSEPR theory accurately predicts that XeF2 is linear.
Note:
It is important to note that nonbonding orbitals exert more repulsions as compared to bonding orbitals because a nonbonding orbital has no nucleus at its far end so as to attract electron cloud towards it and thus, the charge in that orbital is concentrated on the central atom and due to which the repulsions are maximized.
