Question
Question: Does the Lewis structure of \( Br{O_3}^ - \) have three double bonds or two double bonds?...
Does the Lewis structure of BrO3− have three double bonds or two double bonds?
Solution
The Lewis dot structure of any compound is formed by selecting the central carbon atom which is usually the less electronegative atom. The symbols of the side atoms are then written around the symbol of the central atom and different bond formations are highlighted.
Complete answer:
The Lewis dot structure of BrO3− can be drawn by following the steps given below:
The bromine atom is less electronegative and is therefore the central atom.
The oxygen atoms are the side atoms placed around the central atom.
The valence electrons need to be represented by dots in the forms of pairs around bromine and oxygen atoms.
The negative charge stands for an extra electron that goes to the more electronegative atom which is oxygen in this case.
Each shared pair of electrons between two atoms represents a bond.
The structure looks like:
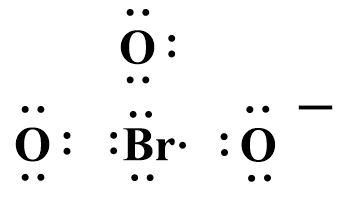
Bromine forms two double bonds with oxygen and satisfies their valency while the third oxygen atom containing a negative charge can only form a single covalent bond.
⇒ Therefore there are only two double bonds in the Lewis dot structure of BrO3− .
Note:
Bromine trioxide ion is a hypervalent chemical species as the central atoms form multiple bonds that result in more than eight electrons around it. The side oxygen atoms simply complete their octet leaving the bromine atom with the extra set of electrons around it.
