Question
Question: Does the copper anode (increases /decreases/remains unchanged) in the weight?  in the weight?
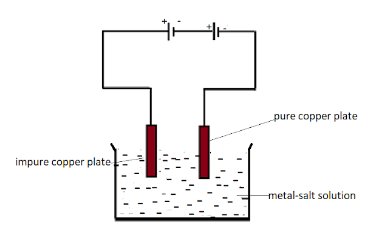
Solution
Hint : Copper plate from the impure copper plate that is the anode of the electrode gets dissolved in the given copper sulphate solution .Copper ions making the anode plate a bit thinner and the copper ions present in the solution gets deposited at the cathode and hence make it thicker so the weight of copper anode decreases.
Complete Step By Step Answer:
Here the given salt solution is copper sulphate salt solution. In the process of electrolysis when the connection is completed the salt solution starts breaking into copper and sulphate ions. The Cu2+ ions are the copper ions that go to the cathode and start depositing on it.
The electrode connected to the negative terminal is the cathode and the positive terminal electrode is the anode which is here impure copper plate. So the copper ions start dissolving in the solution from the anode and then due to positive charged particles they get attracted to the negative terminal that is cathode.
Slowly slowly all the Cu2+ ions gets deposited to the cathode and hence makes the anode thinner.
So the anode copper weight decreases by weight.
Note :
Copper ions make the anode plate a bit thinner and the copper ions present in the solution gets deposited at the cathode and hence make it thicker so the weight of copper anode decreases.
The salt solution should be the same metal salt solution whichever metal is used as electrode to complete the flow of current.
