Question
Question: Does \[LiAl{H_4}\] reduce Amides?...
Does LiAlH4 reduce Amides?
Solution
LiAlH4 is a very strong reducing agent. It reduces aldehydes, amides, ketones, esters, carboxylic acid and even carboxylate salts to alcohols.
Amide is a functional group that contains both nitrogen and carboxyl groups in it. It is usually represented as below:
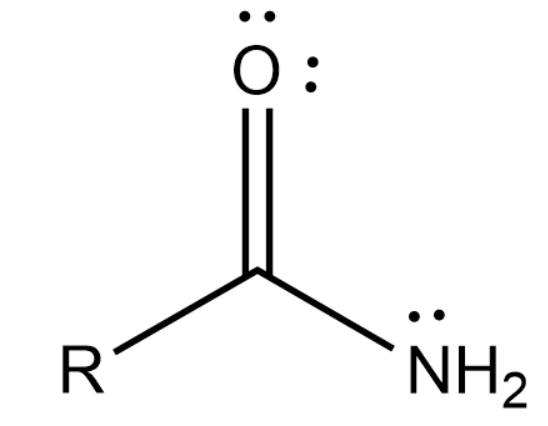
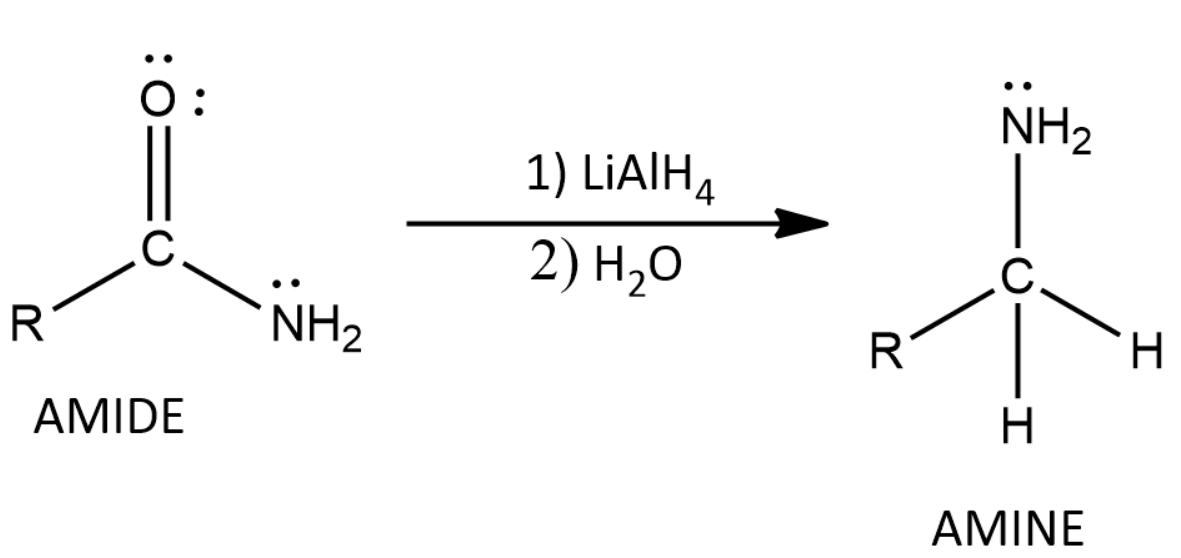
LiAlH4 will reduce Amides to Amines and this means that the oxygen that was present in the amide is removed from the molecule after the reaction completes.
Complete answer:
Let’s see how the reaction occurs when amide is treated with LiAlH4
First there is a nucleophilic attack by one of the hydrides of LiAlH4 . Because the carbonyl group is highly polar the oxygen becomes negative and thus Al having negatively charge can now attack the positively charged carbonyl carbon and thus the following step occurs
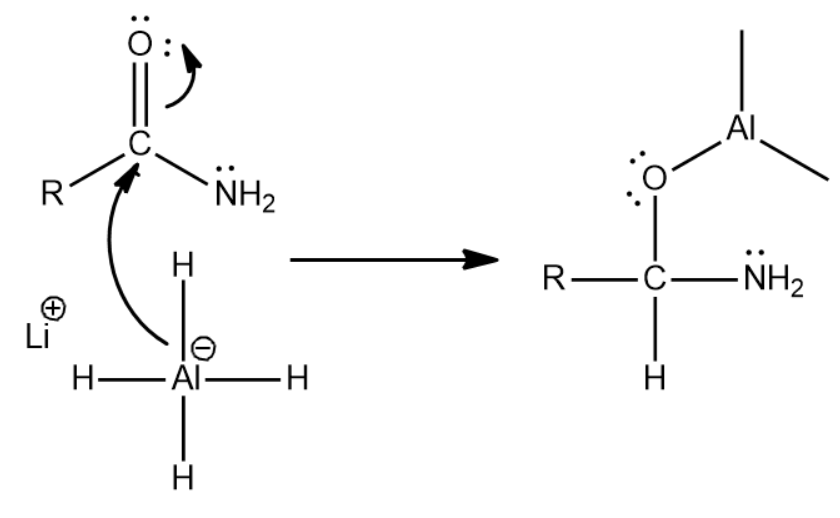
Now since there is an oxygen that can act as a leaving group we will get the following reaction which leads to the formation of an iminium ion
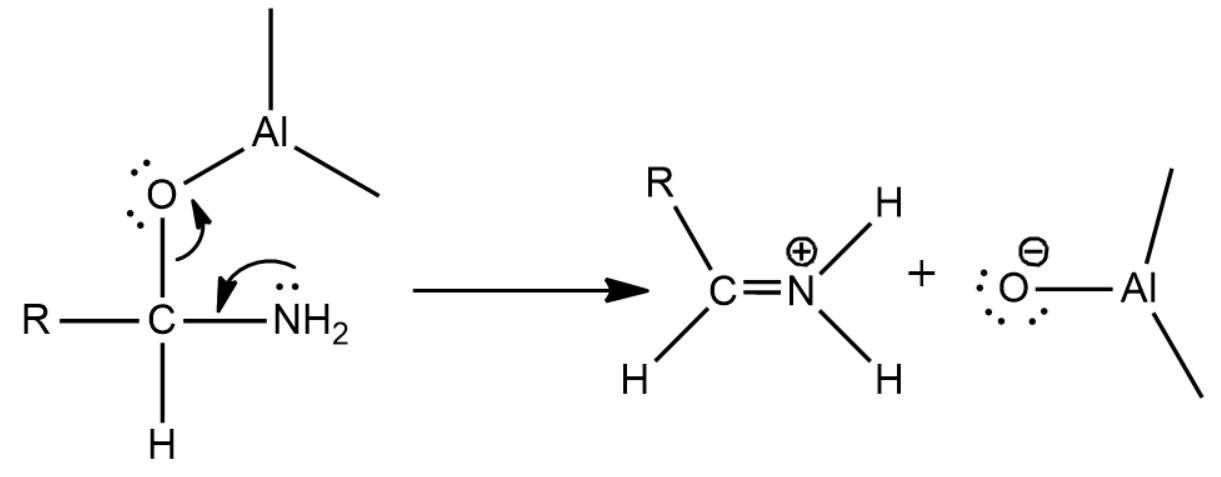
Now there is a nucleophilic attack by the hydride of LiAlH4 causing for the formation of the final product, amine
That is given by the following reaction:
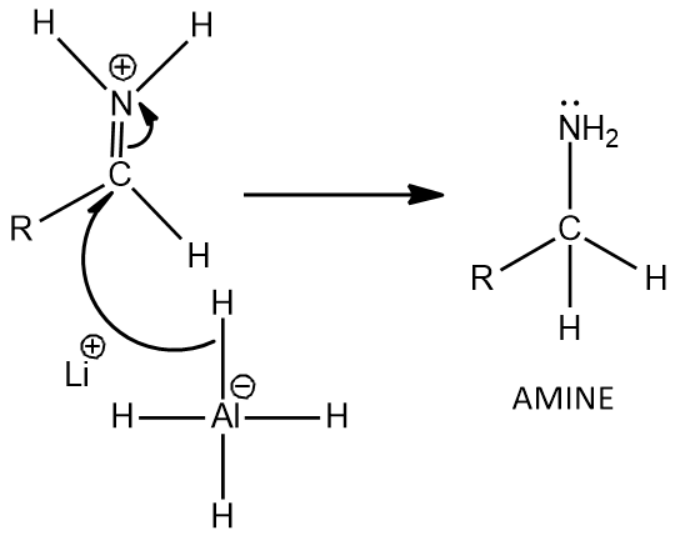
Thus we get Amine as the product when amides are treated with LiAlH4 which is a strong reducing agent.
Thus we can write the general form of the reaction as:
Note:
NaBH4 is also a reducing agent but it is very less reactive than LiAlH4. NaBH4 can reduce aldehydes, ketones and acid chlorides but cannot reduce amides or esters.
Amides can be converted to 1o, 2o or 3oamine just by using LiAlH4. For doing this we have to select the suitable amides to begin the reaction with.
The purpose of using H2O at the end is for a bit of workup. It neutralizes strongly basic reagents at the end of the reaction thus helping in the rate of the reaction.
