Question
Question: Distillation of phenol with zinc-dust gives: (A) Benzene (B) Diphenyl - zinc (C) Diphenyl Ethe...
Distillation of phenol with zinc-dust gives:
(A) Benzene
(B) Diphenyl - zinc
(C) Diphenyl Ether
(D) none of the above
Solution
The product was discovered by Kekule. It has the molecular formula C6H6.
It is an aromatic compound as it obeys Huckel’s rule of (4n+2)πelectrons. It is a very stable compound due to resonance.
Complete step by step answer:
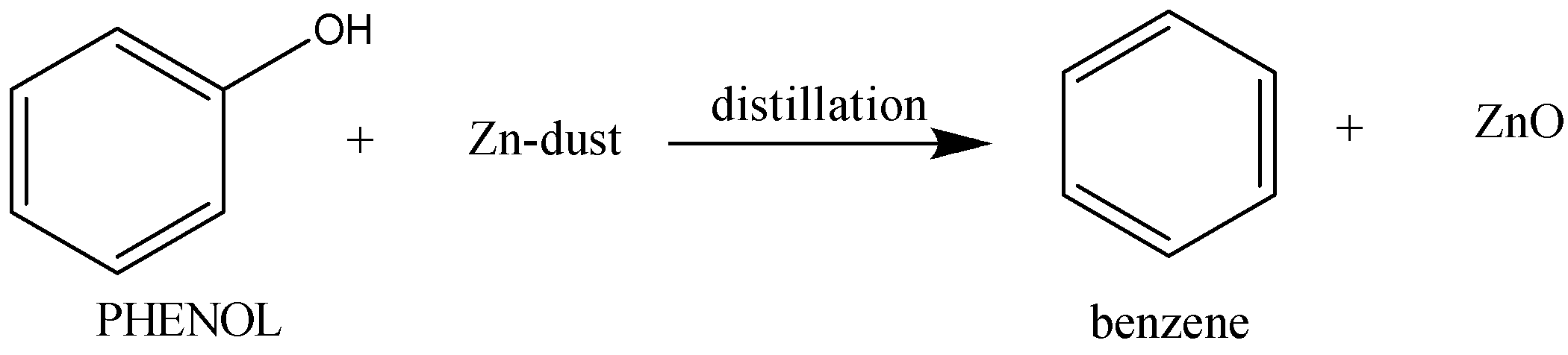
Distillation of phenol with zinc-dust gives benzene and ZnO as a side-product.
The reaction proceeds as follows:
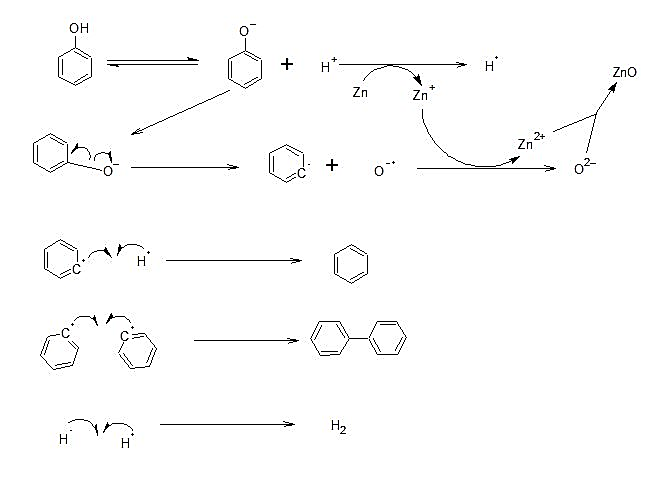
 Detailed Mechanism:
Detailed Mechanism:
- Zn is in the +2 oxidation state.
- The phenol gets converted into phenoxide ion and the released proton accepts an electron from Zn forming hydrogen radical. Due to the heating, there is homolytic fission of C of the phenyl ring andO−.
- ThenO−forms an electron from Zn and forms an oxide ion. In this way, zinc forms zinc oxide, and the phenyl radical produced forms a bond with hydrogen radical to form Benzene and bi-phenyl.
So, the correct option is A.
Note: The yield of this reaction used for benzene formation is lower.
Homolytic fission is the equal splitting of a pair of electrons between two separated atoms.
It is a reduction reaction of phenol to benzene by zinc dust. So, zinc is a reducing agent.
Benzene formed is volatile in nature. It is separated by fractional distillation.
