Question
Question: Dipole moment of which ketone is maximum? (A)  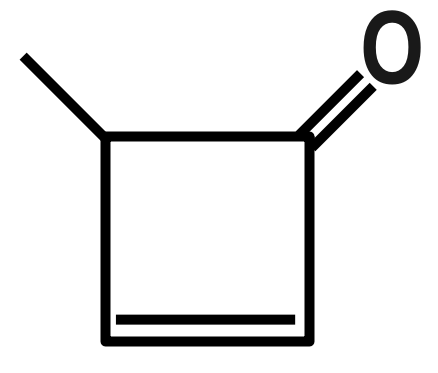
(B) 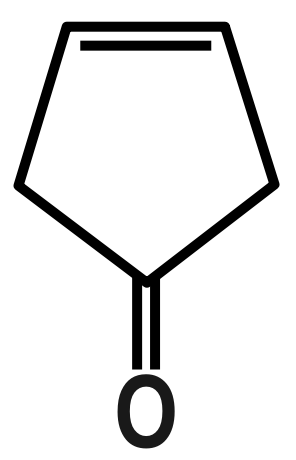
(C) 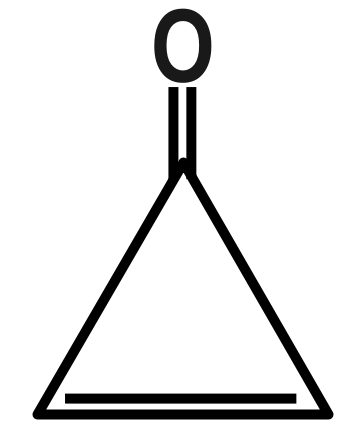
(D)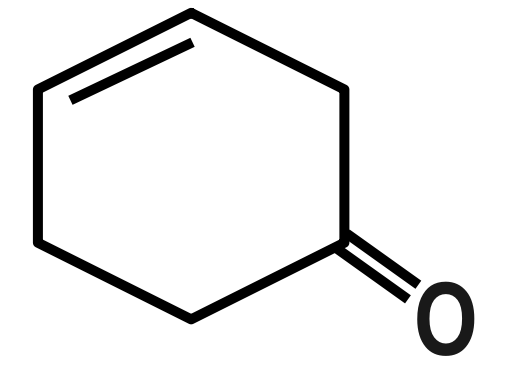
Solution
We know that for calculating the dipole moment we have to consider the magnitude and distance between the charges, but for estimating the highest and lowest dipole moment we can easily find them. If the bonds of carbon are symmetrical that are attached with the same atom it has zero dipole moment but when there is substitution then there is change in dipole moment.
Complete step by step solution:
We know that bonds can be classified as polar and nonpolar because of the electronegativity difference so that polarity is measured in terms of what we call dipole moment. It is defined as the product of magnitude of charge and distance between the charges; mathematically we can write it as
DipoleMoment(μ)=q×d.
Here, is the charge and is the distance between the charges. Now in the above example we have some organic compounds so try to understand their structure as a sphere. So our option is benzene which is symmetrical therefore no dipole moment is there. In the second option we have naphthalene which can be considered as two benzene rings are attached so that’s why we can also see it as spherical and symmetrical.
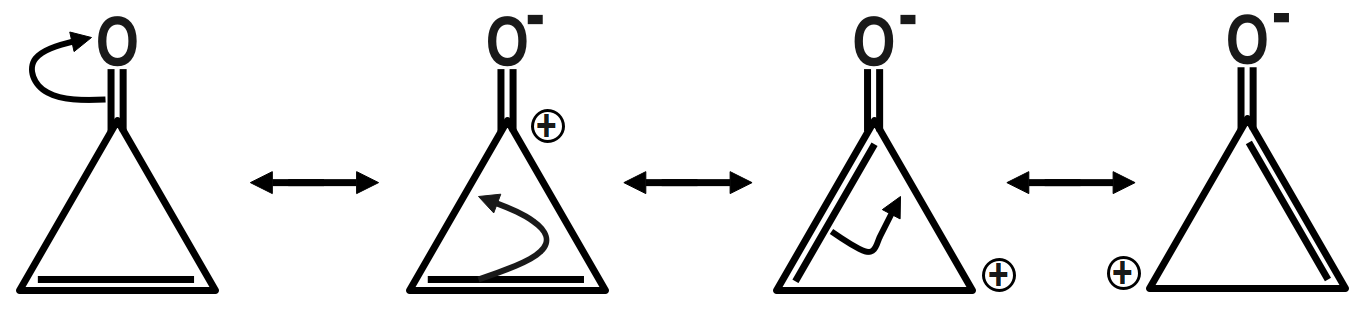
Therefore, the correct answer is option C.
Note:
Remember that Dipole moment has a unit of Debye, which is represented by capital (D). Now there are many examples in which we have to compare between the examples by the only difference we see. See as in options B and D we have a benzophenone type of system but in option B there is little bit difference in the structure by introduction of three membered rings that’s why its dipole moment is greater than benzophenone.
