Question
Question: Dimethyl ether and ethyl alcohol are: A.metamers B.homologues C.functional isomers D.positio...
Dimethyl ether and ethyl alcohol are:
A.metamers
B.homologues
C.functional isomers
D.position isomers
Solution
We know that isomerism is the phenomenon in which two or more compounds have the same chemical formula but different structures. The compounds showing isomerism are known as isomers. There are different types of isomers, such as, position isomers, tautomers, chain isomers etc.
Complete step by step solution:
Let’s first discuss metamers, homologues, functional isomers and position isomers in detail.
Metamers are the isomers which differ with respect to the nature of alkyl groups around a particular functional group. Thus, they belong to the same family. For example, the molecular formula C4H10O possesses three metamers.
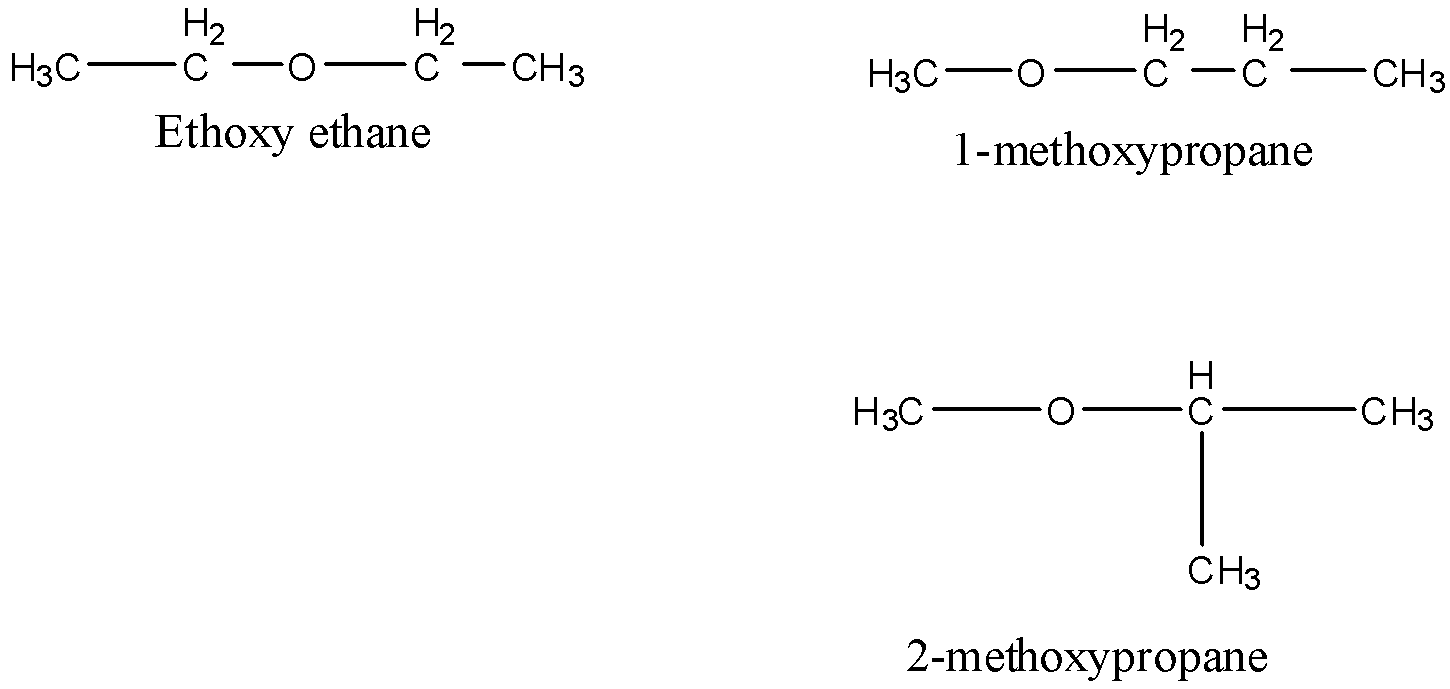
All the isomers belong to the family of ether.
Now, we discuss homologues. Homologues are series of compounds represented by the same molecular formula and a difference ofCH2 group is observed between two successive homologues. Such as, CH4 and C2H6.
Functional isomers are the isomers that differ with respect to the nature of functional groups. So, both the isomers belong to different families. For example, C2H6O has two functional isomers.

Position isomers are the isomers which differ with respect to the position of multiple bonds (double or triple), substituents or functional groups. For example, C3H7OH has two position isomers.
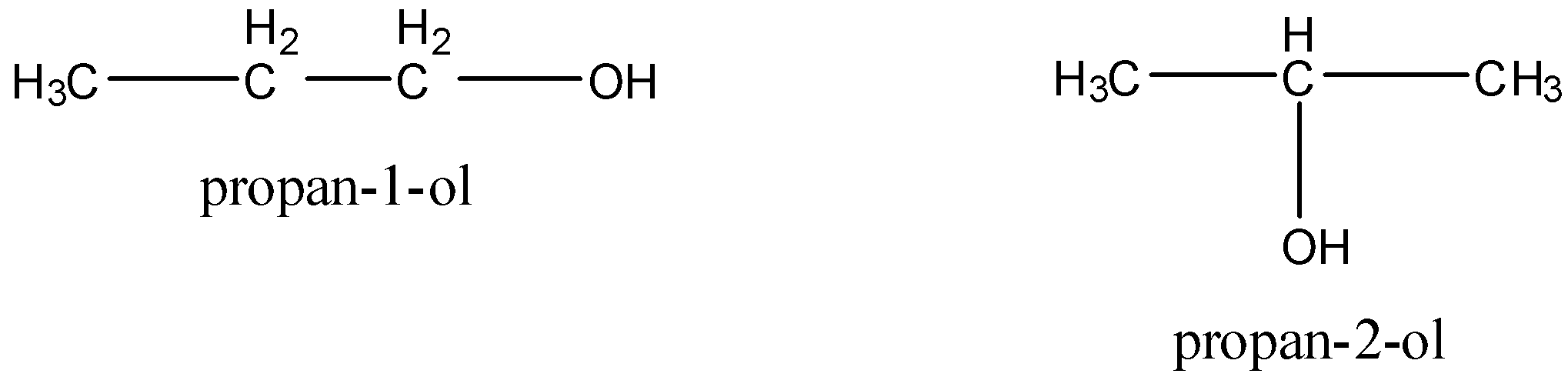
Now, we draw the structure of dimethyl ether and ethyl alcohol.

So, both the compounds differ by their functional groups. So, they are functional isomers.
Therefore, the correct answer is Option C.
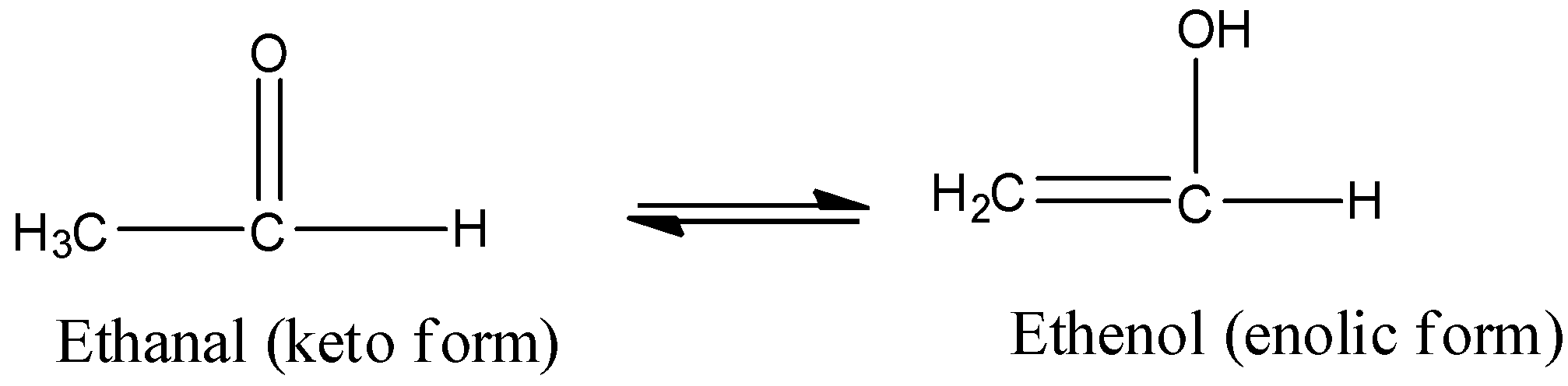
Note: Tautomers are actually functional isomers which exist simultaneously and also in dynamic equilibrium. The isomerism in this case is termed as tautomerism. It is of different types but the most common among them is the keto-enol tautomerism. This isomerism arises due to 1,3 migration of the hydrogen atom from carbon to oxygen atom and vice versa. For example,