Question
Question: Differentiate between primary, secondary and tertiary alcohol victor meyer method....
Differentiate between primary, secondary and tertiary alcohol victor meyer method.
Solution
The primary, secondary and tertiary alcohols are treated as different functional groups and so they are distinguished as they show some different properties and behaviours. Different colours are obtained when these alcohols are made to react with a variety of compounds. These different colours differentiate between the alcohols.
Complete step by step answer:
-Victor Meyer uses laboratory methods to find the molecular weight of a liquid. He also ran a test to distinguish between the primary, secondary and tertiary alcohols with the help of colours given by them when they react in 3 different steps to produce different compounds.
-The alcohols have –OH groups in them. They are made to react with HI, AgNO2 and NaOH in the mentioned sequence only. Alcohol is first converted into iodide when it is made to react with HI by the elimination of water. Then they react with AgNO2 and form different nitroalkanes. These different nitroalkanes react with NaOH and give different colours.
-The reactions for all the alcohols can be shown as:
| Primary alcohol | Secondary alcohol | Tertiary alcohol |
|---|---|---|
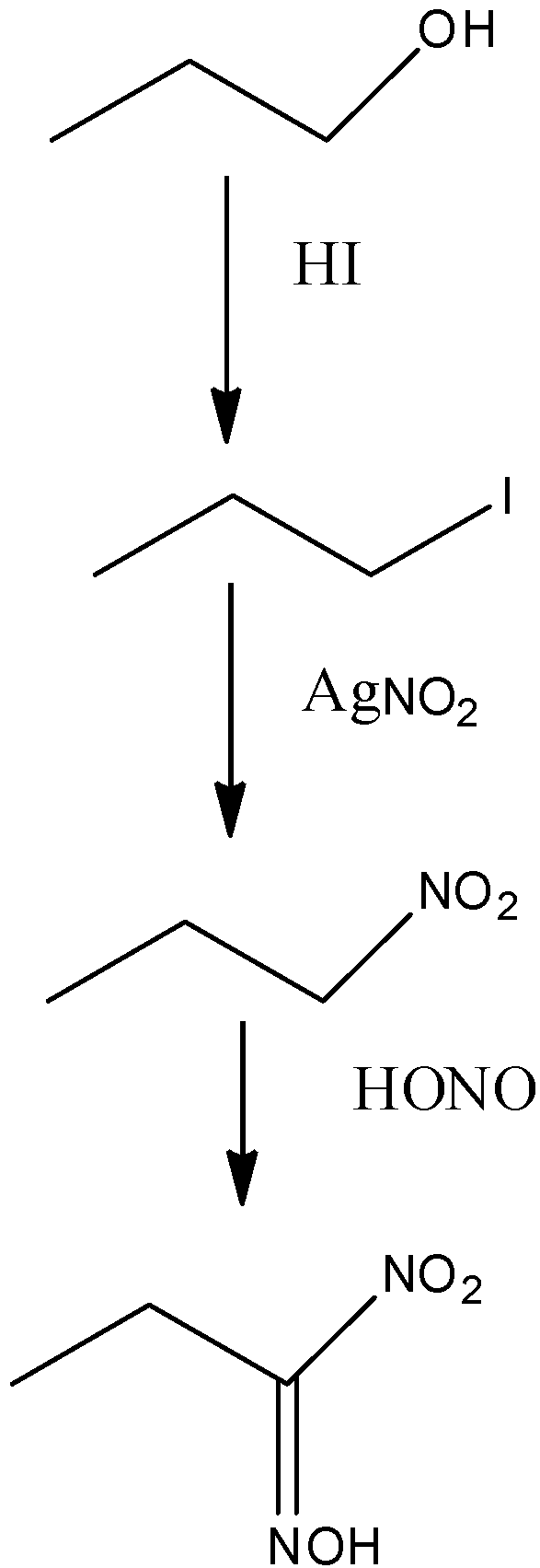 | 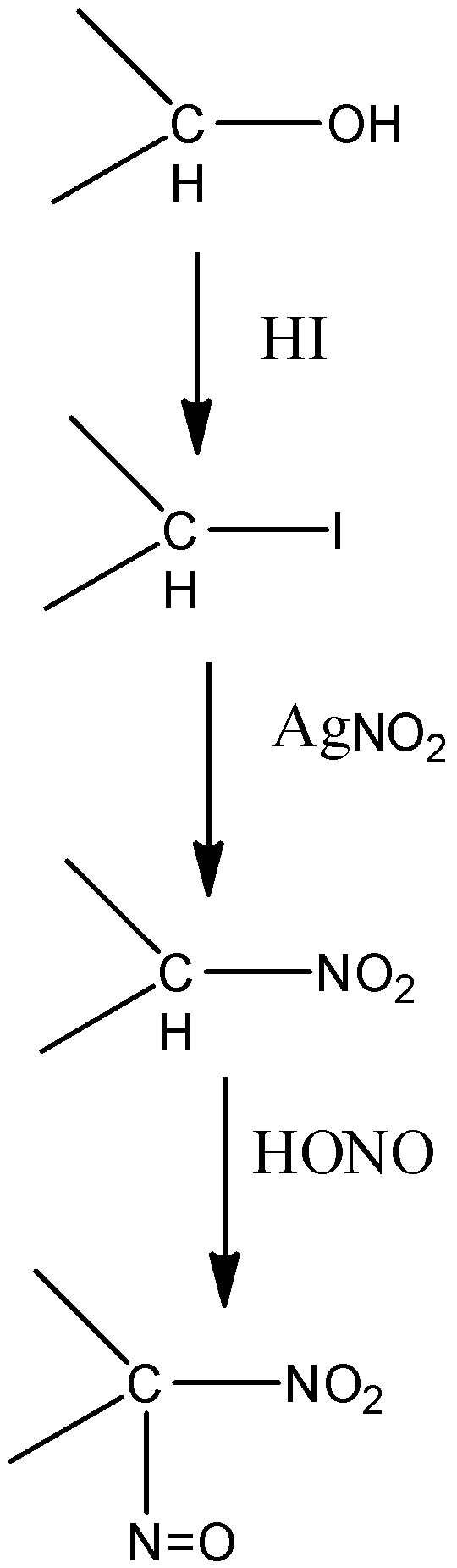 | 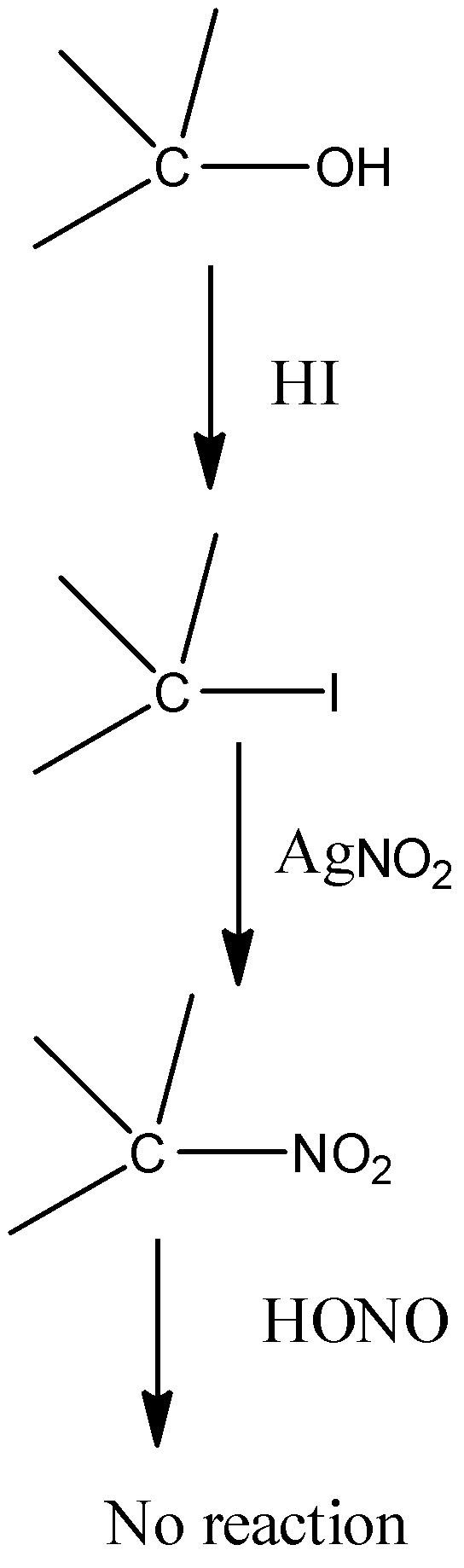 |
| Blood red colour | Blue colour | No colour |
Note: To perform this test, always ensure that the reaction occurs in the given steps only. If the steps are not followed in sequence, then some different products will be formed and the distinguishing test will fail as colours will not be what is desired in the test.
