Question
Question: Difference between shape and geometry of a molecule....
Difference between shape and geometry of a molecule.
Solution
When the atoms of a molecule are arranged in a three-dimensional structure it gives the molecular geometry of that molecule. Various geometrical parameters like bond length, torsional angles, molecular bond angles, etc., that determine the shape of a molecule and the position of the atoms in it.
Complete answer:
When the arrangement of bond pairs and lone pairs around the central atom in a molecule and the coordination number of the molecule is depicted by the geometry of the molecule.
Whereas the lone pairs around the central atom in a molecule are not depicted in the shape of the molecule.
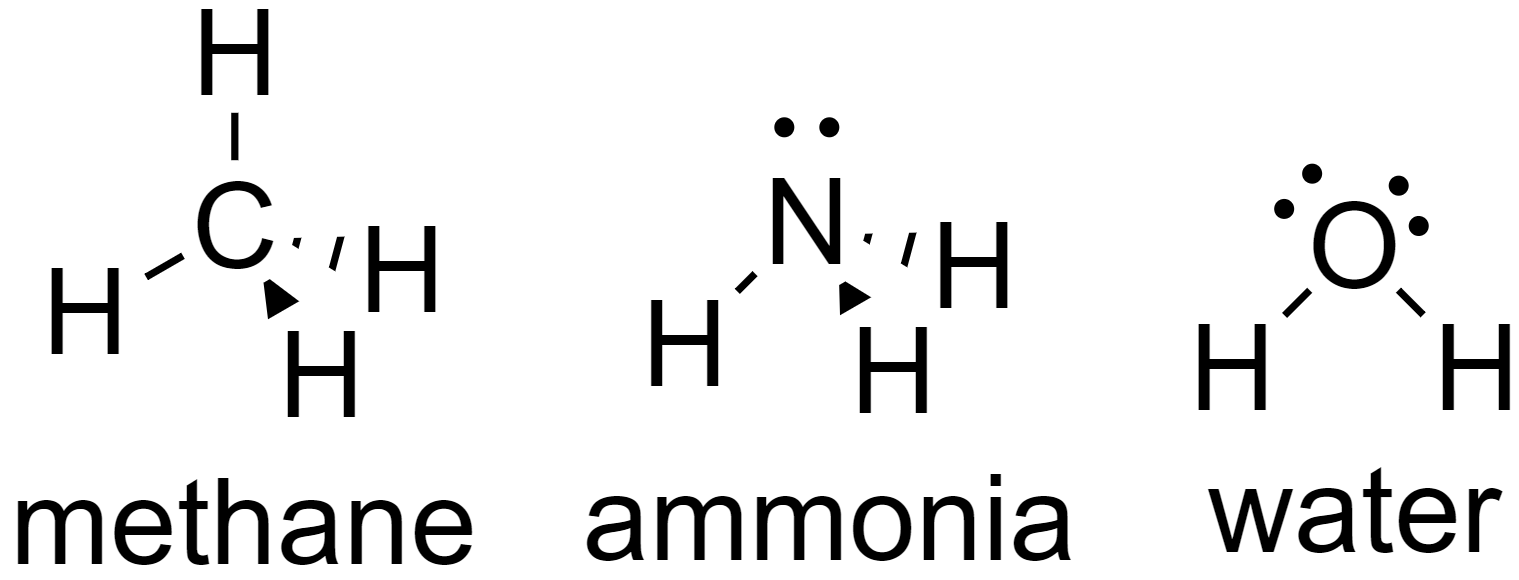
Let us take an example of molecules having coordination number 4, water (H2O), ammonia (NH3), and methane (CH4).
Since all of these molecules have coordination number 4, they all have tetrahedral shapes.
Now due to the presence of lone pairs on water (H2O) and ammonia (NH3), the geometry of these molecules is different whereas since there are no lone pairs on methane (CH4), it will have an equivalent tetrahedral geometry.
Ammonia (NH3) has a trigonal pyramidal shape.
Water (H2O) has a bent v-shape.
Note:
It should be noted that the geometry of a molecule can be determined using the VSEPR model. The VSEPR model of arrangement helps in increasing the stability of the molecule and decreasing its energy.
Now, the formula AXnEm can be used while applying the VSEPR theory to represent the number of electron pairs around a central atom.
Where the central atom is represented by A, ligand bonded to the central atom is represented by X, and lone pairs are represented by E.
Now, the molecular geometry of the compound according to the steric number and the AXnEm formula is
| STERIC NUMBER | AXnEm | MOLECULAR GEOMETRY |
|---|---|---|
| 2 | AX2E0 | Linear |
| 3 | AX3E0 | Trigonal Planar |
| 3 | AX2E1 | Bent |
| 4 | AX4E0 | Tetrahedral |
| 4 | AX3E1 | Trigonal Pyramidal |
| 4 | AX2E2 | Bent |
| 5 | AX5E0 | Trigonal Bipyramidal |
| 5 | AX4E1 | Seesaw |
| 5 | AX3E2 | T-Shaped |
| 5 | AX2E3 | Linear. |
