Question
Question: Difference between \(S{N^{_1}}\& S{N^{_2}}\) reactions...
Difference between SN1&SN2 reactions
Solution
We know that, SN1&SN2 are two types of nucleophilic substitution reactions. A reaction in which an atom or a functional group is replaced by a negatively charged species is known as nucleophilic substitution reaction.
Complete step by step answer: First, we see the SN1 reaction mechanism.
The SN1 reaction:
It is two step reactions, at first step the bond between the carbon and hydrogen breaks hydrolytically and in the second step nucleophile reacts with the carbocation formed in the first step.
The general mechanism of SN1 reaction is,
Step 1: R−XPolarsolventR++X−
Step 2: R++X−[OH]−R−OH
The SN2 reaction:
It is a single step reaction in which the formation of carbocation and leaving of halogen take place simultaneously.
The mechanism of SN2 reaction is,
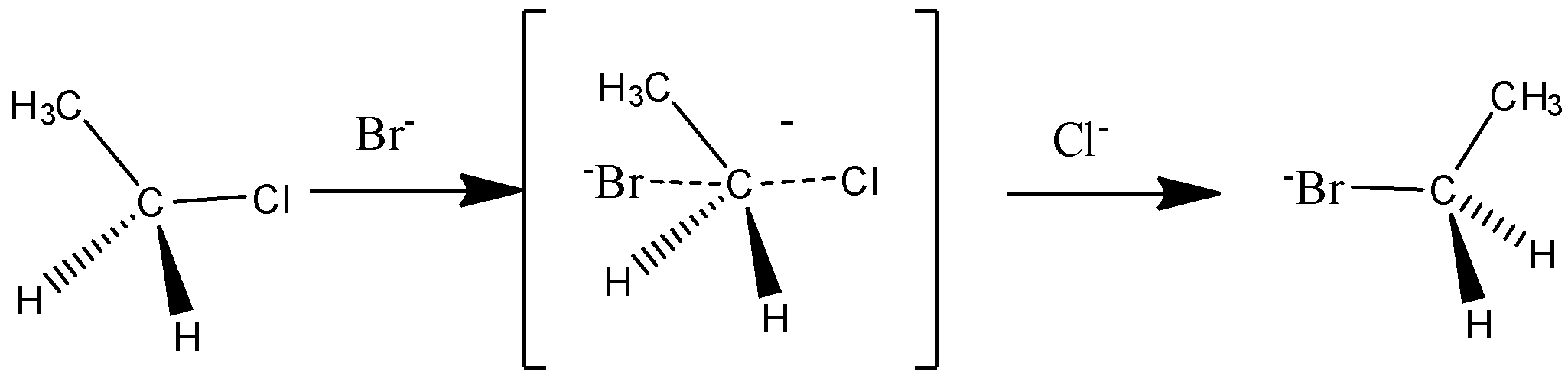
Let us discuss the difference between SN1&SN2 reactions.
| SN1 reactions | SN2 reactions |
|---|---|
| The rate of SN1 reaction is unimolecular. | The rate of SN2 reaction is bimolecular. |
| The rate of the reaction depends on the concentration of the substance, thus it follows first order kinetics. | The rate of reaction depends on the concentration of both nucleophile and substrate. Thus, it follows second order kinetics |
| It is a two step process. | It is a one step process. |
| The intermediate formed is carbocation. | No intermediate is formed during the reaction. |
| No partial bond is formed | Carbon forms a partial bond with the nucleophile and the leaving group. |
| Optically inactive substance becomes optically active. | In SN2 inversion of reaction takes place. |
Note:
We can call electron rich species as nucleophiles; it may be anion, or compound, or atom with at least one lone pair of electrons and the opposite of nucleophile is an electrophile. Electrophile may be a positively charged species.
