Question
Question: Diethyl ether reacts with cold. \[HI\] to give _________ A) Ethyl iodide B) Ethyl alcohol C) ...
Diethyl ether reacts with cold. HI to give _________
A) Ethyl iodide
B) Ethyl alcohol
C) Both A) and B)
D) Ethylene
Solution
The answer to this question is based upon the general concept of chemistry that includes the stability of carbocation, steric hindrance, and the balanced chemical equation that gives the nucleophilic substituted product. This fact leads you to the correct answer.
Complete answer:
We are familiar with the concepts of substitution reactions, addition reactions and also various other types of reactions in the organic chemistry part.
Now, let us see what type of reaction takes place when an ether is made to react with hydrogen iodide.
- In the above question diethyl ether is made to react with cold.HI
The formula for diethyl ether is CH3CH2OCH2CH3
The equation can be written as,
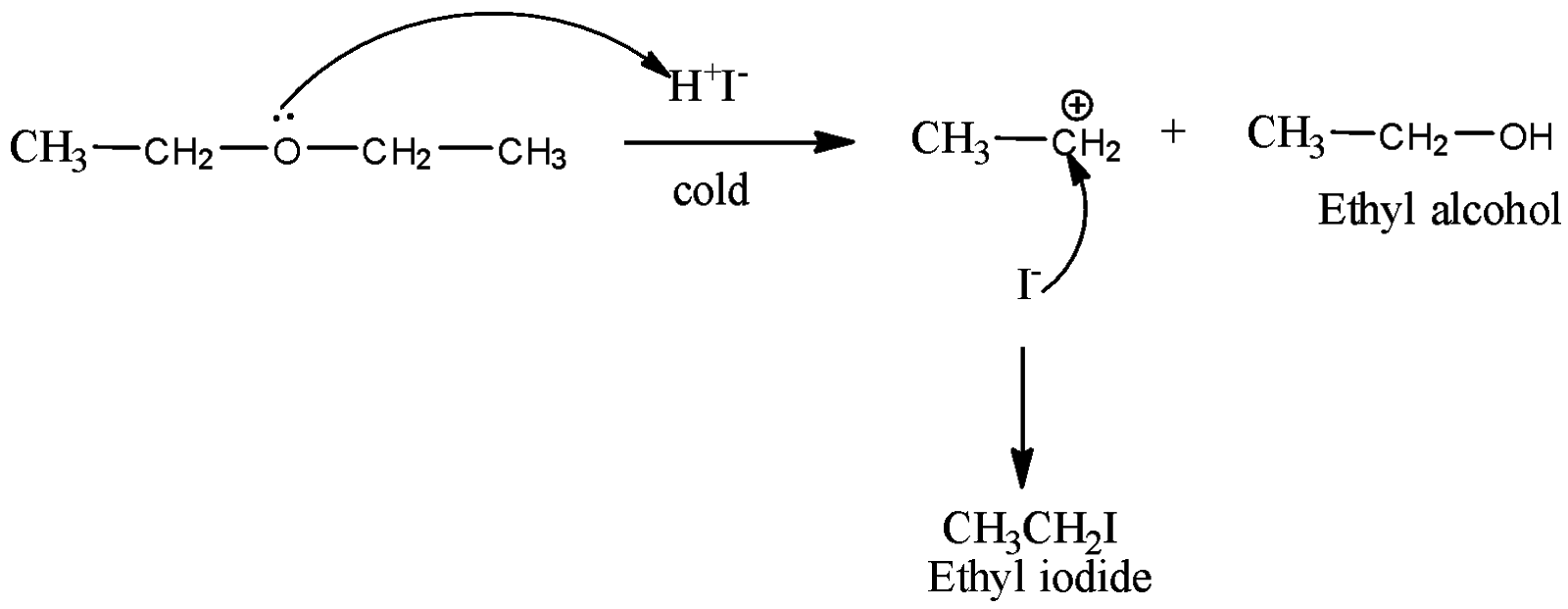
Here, firstly iodine in cold condition dissociates into two ions that is as H+ and I−.
Now, the lone pair present on oxygen atoms attacks the proton of hydrogen iodide and this gives the product as ethyl alcohol which is as shown above.
Now, the nucleophile iodide ion attacks the carbocation which is formed by the breaking of C – O bond and the product forms will be ethyl iodide.
Therefore, the overall products formed when diethyl ether reacts with cold.HIwill be ethyl iodide and ethyl alcohol.
Therefore, the correct answer is option C) Both A) and B).
Note: Note that substitution reaction and addition reactions differ in mechanism where addition reaction occurs when two or more reactants combine together to give a single product which includes all atoms that were present in reactants whereas substitution reaction occurs when there is an exchange of elements in the reactants.
