Question
Question: Diazotization of \[{\text{n - Bu - N}}{{\text{H}}_{\text{2}}}\] with \[{\text{NaN}}{{\text{O}}_{\tex...
Diazotization of n - Bu - NH2 with NaNO2/HCl gives ______ isomeric butene.
A.2
B.3
C.4
D.5
Solution
The chemical process used in converting a primary aromatic amine into the corresponding diazonium salt of the amine is commonly referred to as diazotization. This process is also known as ‘diazotization’.
Complete step-by-step answer: Here reagent given to us is NaNO2/HCl. The reaction between NaNO2 and HCl is as follows:
NaNO2 + HCl→ NaCl + HNO2
The structure of n - Bu - NH2 amine is as follows:
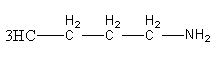
Diazotization of n - Bu - NH2 with NaNO2/HCl gives diazonium ion as the intermediate product.
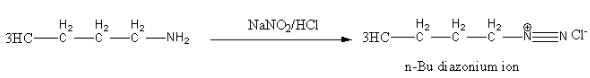
Alkyl diazonium salts are unstable so converted into carbocation. In this reaction N2 is a good leaving group.
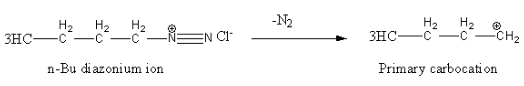
This primary carbocation converted into 1-butene after the loss of hydrogen as follows.
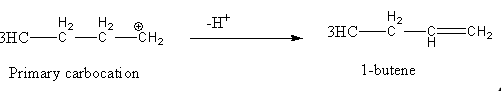
So, we can say that Diazotization of n - Bu - NH2 with NaNO2/HCl gives 1-butene as one of the products.
Primary carbocation also shows rearrangement of the hydrogen atom and converts into secondary carbocation as follows:

This secondary carbocation converted into 2-butene after the loss of hydrogen as follows
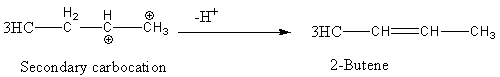
2-butene has two types of isomers cis and trans.
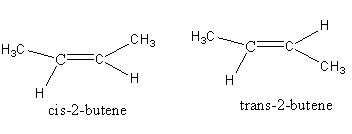
Diazotization of n - Bu - NH2 with NaNO2/HCl gives 1-butene, cis-2-butene and trans-2-butene isomers.
So, we can say that diazotization of n - Bu - NH2 with NaNO2/HCl gives 3 isomeric butene.
Hence, the correct option is (B) 3
Note: Isomer having similar groups on the same side is known as a cis isomer. Isomers having similar groups on opposite sides are known as the trans isomer. Cis and trans isomers are known as a geometrical isomers. Alkenes having two different substituents at each end of C = C show geometrical isomers. 1-butene does not show any isomer.
