Question
Question: Diamonds and Graphite are the two forms of which non-metal?...
Diamonds and Graphite are the two forms of which non-metal?
Solution
Between non-metal atoms, covalent bonds develop. Each connection is extremely strong and consists of a shared pair of electrons. The characteristics of simple molecular compounds and massive covalent structures are vastly different. Carbon is a chemical element with the atomic number 6 and the symbol C. It's nonmetallic and tetravalent, which means it has four electrons available for chemical bonding.
Complete answer:
Allotropes of pure carbon include diamond, graphite, and fullerenes (substances that include nanotubes and buckyballs, such as buckminsterfullerene). The carbon atoms are linked by strong covalent bonds in all three allotropes, but in such diverse configurations that the characteristics of the allotropes are vastly different.
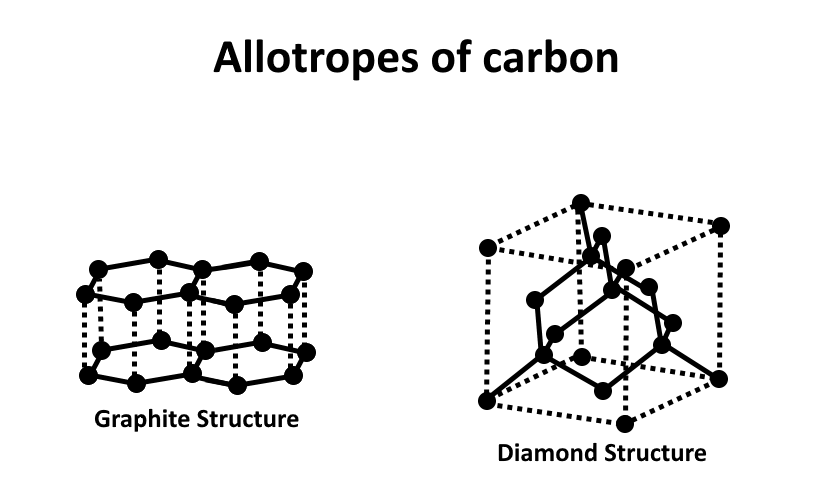
Diamond
A diamond is a massive carbon atom molecule. Diamonds are clear and colourless. They are regarded as glossy because they shine and reflect light. These characteristics make them appealing in jewellery.
It has a high melting point and is highly hard. As a result, it is extremely helpful in cutting tools. Diamonds are used in heavy-duty drill bits, such as those used in the oil exploration sector to drill through rocks, so they stay sharper for longer. Water does not dissolve diamond. It is not an electrical conductor. A diamond has no free electrons or ions since each atom is linked to its neighbours by four strong covalent bonds. This explains why diamond is not an electrical conductor. The bonding also explains why diamond is so hard and has such a high melting temperature. To separate atoms that are so tightly bound together, large amounts of energy would be required.
Graphite
Carbon atoms are arranged in layers in graphite. Graphite is a black, lustrous, and opaque material. It's not see-through. It's a really slick substance. Layers readily glide onto the paper, producing a black trace, therefore it's utilised in pencil leads. It is insoluble. It has a high melting point and is an excellent conductor of electricity, making it an ideal material for electrolysis electrodes. Three strong covalent connections bind each carbon atom to its layer. Each atom now has an extra electron, forming a delocalized sea of electrons that loosely bonds layers together. Because these delocalized electrons may all travel in the same direction, graphite is an excellent electrical conductor.
Note:
Allotropy refers to the ability of some chemical elements to exist in two or more distinct forms in the same physical state, which are referred to as allotropes of the elements. Allotropes are various structural variations of an element in which the atoms are linked in a different way.
