Question
Question: Diamond is hard because: A. All the four valence electrons are bonded to each carbon atoms by cova...
Diamond is hard because:
A. All the four valence electrons are bonded to each carbon atoms by covalent bonds
B. It is a giant molecule.
C. It is made up of carbon atoms
D. It cannot be burnt
Solution
Hint: Diamond is an allotropic form of carbon. Diamond is a solid form of the carbon element with its atoms arranged in a crystal structure called diamond cubic. At room temperature and pressure, another solid form of carbon element known as graphite is the chemically stable form.
Complete step by step answer:
We know that in diamond each carbon atom is linked to four other carbon atoms by a bond called sigma bond. Each sigma bond is formed by the overlapping of sp3hybrid orbitals of each carbon atom. Each carbon atom is located at the center of a regular tetrahedron and is enclosed by four other carbon atoms present at the corners of a regular tetrahedron.
In diamond the octet of each carbon atom is completed. Structure of the diamond is a rigid three dimensional network. This causes high density and hardness to the diamond. Diamond is chemically inactive due to its rigid three dimensional structure. Bonding of carbon in diamond is as follows.
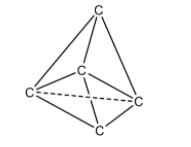
High energy is required to break the large number of sigma bonds present in diamond. This is the reason for the high melting point of the diamond.
So, the correct option is A.
Note: Don’t be confused with the terms Diamond and Graphite. Both are not the same.
In diamond the hybridization of the carbon is sp3and a bad conductor. But in case of graphite the hybridization is sp2, due to sp2 hybridization graphite acts as a semiconductor.
