Question
Question: Determination of the molar mass of acetic acid in benzene using freezing point depression is affecte...
Determination of the molar mass of acetic acid in benzene using freezing point depression is affected by:
A. complex formation
B. association
C. partial ionization
D. dissociation
Solution
The freezing point depression is a colligative property. The value of freezing point depression depends solely on the number of ions or components present on the system.
Complete step by step answer:
The given question is related to the colligative property of solutions. The freezing point depression is the lowering of freezing point of solvent due to addition of solute particles. The depression in freezing point (ΔTf) is expressed as
ΔTf=iKfm
Where i is the van’t Hoff factor, Kf if the cryoscopic constant and m is the molality.
The depression in freezing point depends on the factor i which is the number of ions or components and the molality. The molality is the number of moles of acetic present per kg of the solvent. It is related to the molar mass of acetic acid.
The value of I depends on the fate of the solute added to the solvent system. The fate of solute decides whether the solute undergoes any dissociation or association with the solvent molecules. Due to such changes in solution the number of ions present in the solution changes and so the value of freezing point depression (ΔTf) changes.
The solvent given here is benzene which is a nonpolar solvent and the solute is acetic acid which is a polar molecule. These two components do not undergo any interaction like dipole-dipole or hydrogen bonding with each other. But the acetic acid itself is able to undergo interaction with another acetic acid molecule.
This results in association of the acetic acid molecules due to hydrogen bonding interaction. Hence the actual number of acetic acid molecules decreases in the solution. The hydrogen bonding interaction is shown as:
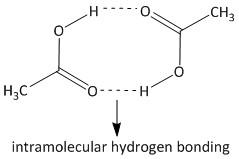
Due to the presence of such type of intramolecular interaction the actual number of molecules of acetic acid decreases to half. So the freezing point depression is erroneous. Hence the determination of molar mass of acetic acid is affected by association, i.e. option B is the correct answer.
Hence option (B) is the correct answer.
Note:
The number of molecules should not be confused with the number of moles of solute. The freezing point depression does depend on the concentration of the solute and only on the total number of solute components in the mixture of solute and solvent. Unlike association, dissociation of a solute particle results in an increase of the number of molecules in the solution and as a result colligative property changes.
