Question
Question: Designate whether each of the following compounds is aromatic or not aromatic....
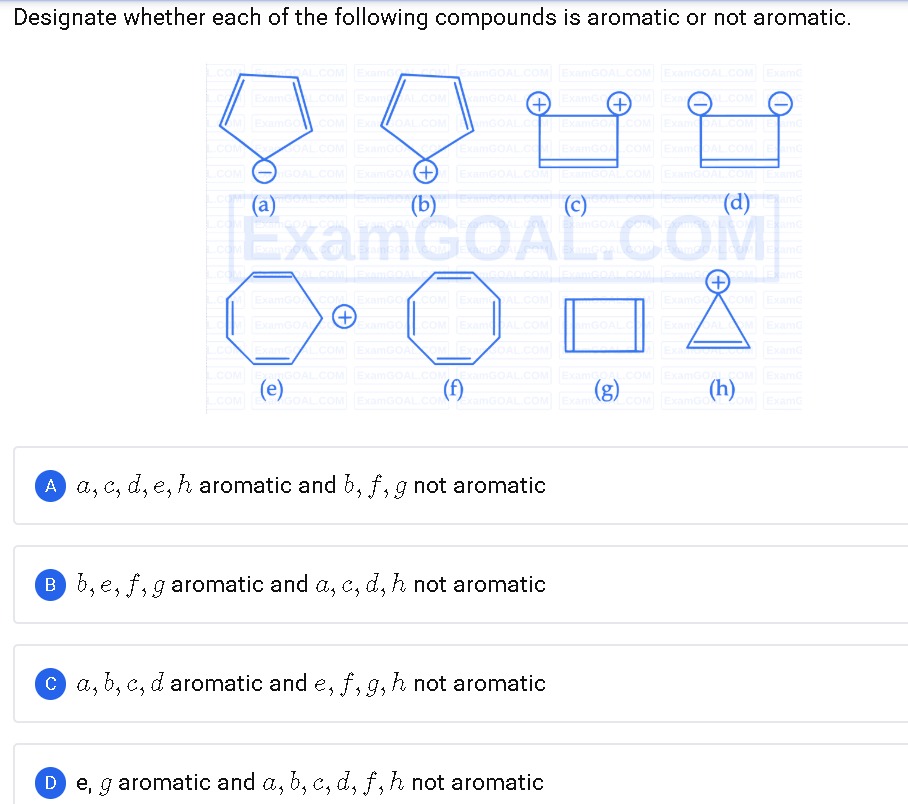
Designate whether each of the following compounds is aromatic or not aromatic.
A
a, c, d, e, h aromatic and b, f, g not aromatic
B
b, e, f, g aromatic and a, c, d, h not aromatic
C
a, b, c, d aromatic and e, f, g, h not aromatic
D
e, g aromatic and a, b, c, d, f, h not aromatic
Answer
a, c, d, e, h aromatic and b, f, g not aromatic
Explanation
Solution
Aromaticity is determined by planarity, cyclic conjugation, and Hückel’s rule (4n+2 π electrons).
- (a) Cyclopentadienyl anion: 6 π electrons → aromatic.
- (b) Cyclopentadienyl cation: 4 π electrons → antiaromatic → not aromatic.
- (c) Cyclobutadiene dication (2 π electrons): meets Hückel’s 4n+2 with n=0 → aromatic.
- (d) Cyclobutadiene dianion (6 π electrons): planar 4‑membered with two lone pairs → aromatic.
- (e) Cyclooctatetraenyl cation: 6 π electrons → aromatic.
- (f) Neutral cyclooctatetraene: 8 π electrons, adopts nonplanar tub conformation → not aromatic.
- (g) Cyclobutane: saturated ring, no continuous π system → not aromatic.
- (h) Cyclopropenyl cation: 2 π electrons → aromatic.
