Question
Question: Describe with diagram the laboratory method of preparation of ethylene. Write its chemical reaction ...
Describe with diagram the laboratory method of preparation of ethylene. Write its chemical reaction with the following. Also write the relevant chemical equations:
(i) Baeyer's reagent
(ii) Ozone
(iii) Sulphur monochloride
(iv) Chlorine
Solution
Ethylene is a hydrocarbon having the chemical formula of or . It is a colourless and flammable gas and possesses a faint "sweet or musky" odour in its pure form. It is considered to be the simplest alkene.
Complete answer:
The dehydration of ethanol in the presence of either concentrated sulphuric acid or concentrated phosphoric acid at laboratory-scale leads to the production of ethylene as shown in the reaction below:
CH3−CH2−OHconc.H2SO4−H2OCH2=CH2
This laboratory method of preparation of ethylene can be better understood from the schematic diagram demonstrated below:
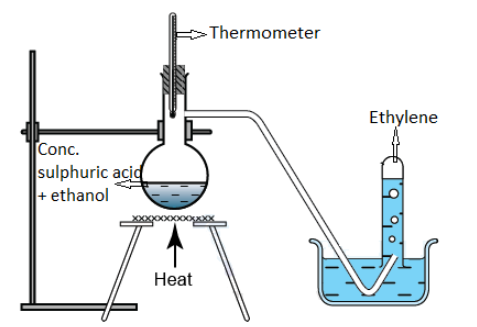
The relevant chemical reactions of ethylene are written below with different reactants:
(i) Baeyer's reagent: It is an alkaline solution of the cold potassium permanganate (powerful oxidant). When Ethylene reacts with Baeyer's reagent, ethylene glycol is produced. The colour of the solution changes from pink to colourless.
CH2=CH2alkalineKMnO4Δ2HCOOH→2CO2+2H2
(ii) Ozone: When Ethylene reacts with ozone, initially ozonide of ethylene is formed which then reacts with Zn/H2O to finally form methanal as shown below:
CH2=CH21)O32)Zn/H2O2HCHO
(iii) Sulphur monochloride: Ethylene reacts with Sulphur monochloride to yield mustard gas or sulphur mustard (C4H8Cl2S) as shown below:
2H2C=CH2+S2Cl2→S(−CH2−CH2−Cl)2+S
(iv) Chlorine: When ethylene reacts with chlorine, Dichloroethane is produced as shown below:
H2C=CH2+Cl2→C2H4Cl2
Note:
There are multiple applications of ethylene. Ethylene is generally used as an anaesthetic in hospitals. It is also used as an oxy-fuel gas in welding, metal cutting, and high velocity thermal spraying. Other uses of ethylene include it is utilised as a refrigerant and also employed in the extraction of rubber.
