Question
Question: Describe the laboratory method of preparation of sulfur dioxide giving chemical equations. Give chem...
Describe the laboratory method of preparation of sulfur dioxide giving chemical equations. Give chemical equations of the reactions of sulfur dioxide with potassium permanganate and lime water.
Solution
Sulfur dioxide used in winemaking from the empty wine glass smelled fresh on burning sulfur candles inside the glass. The chemical formula of this compound is SO2 and exists in small concentrations in the earth’s atmosphere but the most abundant gas in the atmosphere of Venus.
Complete step by step solution:
Sulfur dioxide is commonly referred to as sulfuric acid anhydride, sulfurous anhydride, or sulfur oxide. The structure of sulfur dioxide is bent molecule as shown below with bond order 1.5
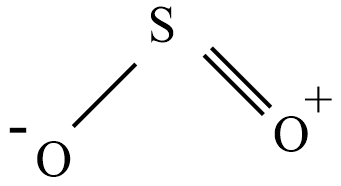
Preparation of sulfur dioxide:
(i) In the laboratory, sulfur dioxide is prepared from the reaction of metallic bisulfate like sodium bisulfate with dilute acid like dilute sulfuric acid.
Na2SO3+H2SO4→Na2SO4+H2O+SO2
(ii) When Cu turnings are heated with concentrated sulfuric acid in the laboratory method.
Cu+2H2SO4→CuSO4+SO2+2H2O
Chemical properties of sulfur dioxide:
(i) When sulfur dioxide reaction with potassium permanganate, decolorization of pink permanganates solution
2SO2+KMnO4+2H2O→K2SO4+H2SO4+MnO2
(ii) When sulfur dioxide passes through lime water, white precipitate (calcium sulfite) is formed.
SO2+Ca(OH)2→CaSO3↓+H2O
Note: Sulfur dioxide is a colorless gas with a rotten egg smell. It liquefies easily and highly soluble in water. It is a strong oxidizing agent but does not support the combustible reactions. Both Carbon dioxide and sulfur dioxide turns lime water milky, but carbon dioxide is an odourless gas and sulfur dioxide has a characteristic smell.
