Question
Question: Derive the formula for efficiency of the Carnot engine....
Derive the formula for efficiency of the Carnot engine.
Explanation
Solution
Carnot engine was proposed by Nicolas Leonard sadi Carnot in 1824. Carnot engine which gives an estimate of the maximum possible efficiency of a heat engine during the conversion process of heat into work. A carnot engine is used in heat pumps and working fluid has a fixed mass throughout a cycle.
Complete step-by-step solution:
Carnot cycle consist of following four processes and carnot engine is an ideal reversible closed thermodynamic cycle
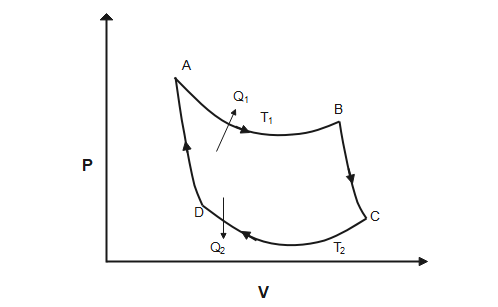
- Isothermal expansion
Heat Q1 is absorbed from the reservoir at temperature T1 and gas is taken from P1,V1,T1 to P2,V2,T2. The total change in internal energy is zero and work done by the gas on the environment is equal to the heat absorbed by the gas.
Work done by the gas is given by:
W12=Q1=μRT1×lnv1v2 - Adiabatic expansion
From P2,V2,T1 to P3,V3,T2 gas expands adiabatically
Work done by the gas is given by:
W23=γ−1μR(T1−T2) - Isothermal compression:
From P3,V3,T2 to P4,V4,T2 gas is compressed isothermally
Work done by the gas is given by:
W34=μRT2lnv4v3 - Adiabatic compression
From P4,V4,T2 to P1,V1,T1 gas is compressed adiabatically
Work done by the gas is given by:
W41=γ−1μR(T1−T2)
Total work done by the gas in one complete cycle is given by:
