Question
Question: Dehydration of alcohols takes place more rapidly with \(POC{l_3}\) than with \({H_2}S{O_4}\). Select...
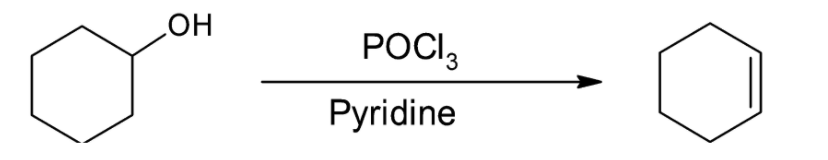
Dehydration of alcohols takes place more rapidly with POCl3 than with H2SO4. Select the correct statements about the above dehydration reaction:
A.It does not involve carbocation
B.It involves R−OPOCl2 with −OPOCl2 as a better leaving group
C.It involves the E2 mechanism as the pyridine base abstracts protons from the adjacent carbon at the same time at which −OPOCl2 is leaving.
D.It is an E1 reaction without formation of carbocation.
Solution
Dehydration of alcohols is a method for the preparation of alkenes from alcohols. POCl3 is a very useful reagent for the dehydration of alcohols for the production of alkenes. It has an advantage over dehydration using H2SO4 as unlike the latter, no rearrangement occurs in the reaction with POCl3.
Complete answer:
The dehydration reaction given is occurring in the presence of POCl3 as initial attacking species and pyridine as base. Phosphorous oxychloride is a derivative of phosphoric acid and converts the alcohol into an alkyl chloro phosphite. The −OPOCl2 group formed is a strong leaving group as compared to the hydroxyl group, thus making the reaction more feasible.
Now, the base pyridine attacks the intermediate alkyl phosphate and deprotonates it. The reaction occurs by the E2 mechanism and no carbocation is formed. The hydrogen ion is abstracted in the same step as the chloro phosphate group leaves.
Thus, we can conclude that the correct options are A, B and C.
Note:
We know that the hydroxyl group is a very poor leaving group. This is because the bond formed between carbon and oxygen is very strong due to almost similar sizes of the atoms and thus it is difficult to cause the removal of the hydroxyl group by breaking the carbon- oxygen bond. So, to make sure that the alcohols participate in a desired substitution or elimination reaction which involves the departure of the hydroxyl group, the hydroxyl group is first modified so as to stabilize the anion formed on the cleavage of carbon- oxygen bond thus making the cleavage more feasible.
