Question
Question: Degree of unsaturation of \( A=2 \) and it contains no double or triple bonds. Identify the structur...
Degree of unsaturation of A=2 and it contains no double or triple bonds. Identify the structure of A
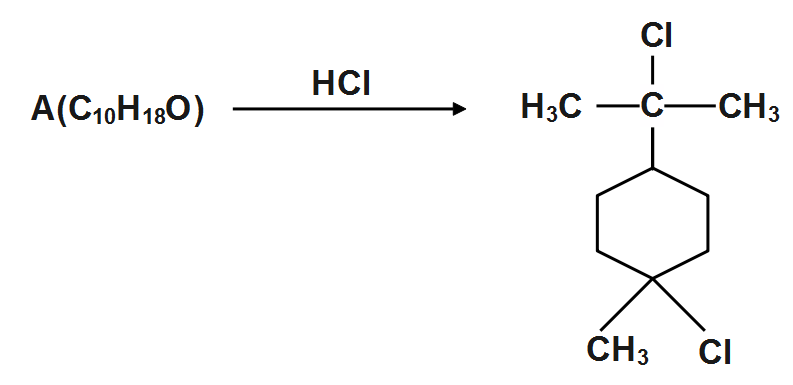
(A) 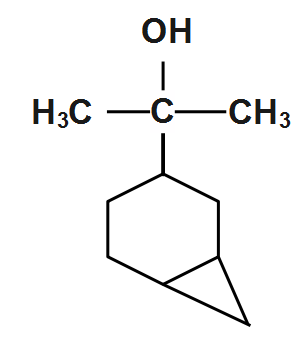
(B)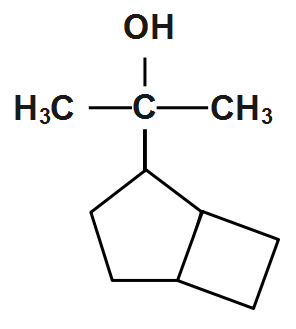
(C)
(D) None of these.
Solution
Hint : We know that the ketone is a structure with R2C=O configuration and in this R can be a variety of carbon-containing substituents. They are a carbonyl group which have a carbon-oxygen double bond. The simplest of ketone is called acetone. Ketones have a lot of industrial importance such as great importance in biology. Such as many sugars, steroids and solvent acetone.
Complete Step By Step Answer:
The carbon is ketone has sp2 hybridization and that includes both their electronic and molecular structure. They are trigonal planar in structure and differ from aldehyde in that the carbonyl group O is bonded to two carbon within a carbon skeleton. Ketones are also distinct from other carbonyl-containing functional groups, such as carboxylic acids, esters and amides
Carbonyl groups are polar as the electronegativity of oxygen is more than that of carbon. This difference causes the polarity to arise. This means ketones are nucleophilic at oxygen and electrophilic at carbon. Ketones are water soluble and are hydrogen bond acceptors. They are not hydrogen-bond donors and thus cannot hydrogen-bond to themselves.
We can see that the degree of saturation is 2 but it contains no double or triple bond. Thus we can see that there are two rings-one six membered as indicated by product and the other three membered which is cleaved by hydrochloric acid due to strain. This A has given the structure.
Note :
Note that the oxidation of hydrocarbons often with air is an important method known for ketone production. Cyclohexanone is produced annually by aerobic oxidation of cyclohexane. The preparation of Acetone occurs by air-oxidation of cumene. Because of their inability to serve both as hydrogen-bond donors and acceptors, ketones tend not to "self-associate" and are more volatile than alcohols and carboxylic acids of comparable molecular weights. Because the carbonyl group interacts with water by hydrogen bonding.
