Question
Question: Define the terms (i) ‘cut-off voltage’ and (ii) ‘threshold frequency’ in relation to the phenomenon ...
Define the terms (i) ‘cut-off voltage’ and (ii) ‘threshold frequency’ in relation to the phenomenon of photoelectric effect.
Using Einstein’s photoelectric equations show how the cut-off voltage and threshold frequency for a given photosensitive material can be determined with the help of a suitable plot/ graph.
Solution
Hint: Photoelectric current flows through the circuit when photoelectrons are ejected. If we apply reverse voltage then photocurrent decreases. Photoelectrons are ejected only when the light of sufficient energy falls on the metal surface and the energy of light is dependent on its frequency.
Complete step by step answer:
Photoelectric effect:
When the light of sufficient energy falls on the metal surface it knocks out the outermost electrons from the metal. This phenomenon is called the photoelectric effect. The knocked electrons are called ‘Photoelectrons’.
The minimum energy required to knock out electrons from the metal surface is called the work function () of that metal. If the light has energy more than the work function of metal falls on it then the rest of the energy is converted into kinetic energy of the electron.
Therefore, when a light of a certain frequency on a cathode has a work function then the kinetic energy of the ejected electron is given by Einstein’s photoelectric equation.
K.E.=hν−φ
Where h is Planck’s constant and is the energy of the incident light.
These ejected photoelectrons are collected by anode terminal hence a photoelectric current flows in a circuit. If we apply a reverse voltage making the anode negative then the photocurrent in the circuit decreases. The reverse voltage for which photocurrent becomes zero is called ‘cut off voltage’.
The photoelectrons are not ejected for the light of all wavelengths or frequencies. There is a minimum frequency for which photoelectrons are just ejected with zero kinetic energy. This minimum frequency of light is called ‘threshold frequency’. At this frequency, the cut off voltage is zero.
If V is the voltage applied to between cathode and anode then kinetic energy is given as
K.E.=eV
Where e is the charge on electron.
Therefore,
eV=hν−φ
Forν=ν0, threshold frequency, V=0,
Therefore,
hν0=φ
So Einstein’s photoelectric relation will be
eV=hν−hν0V=ehν−ehν0
This relation is of the form y=mx+c
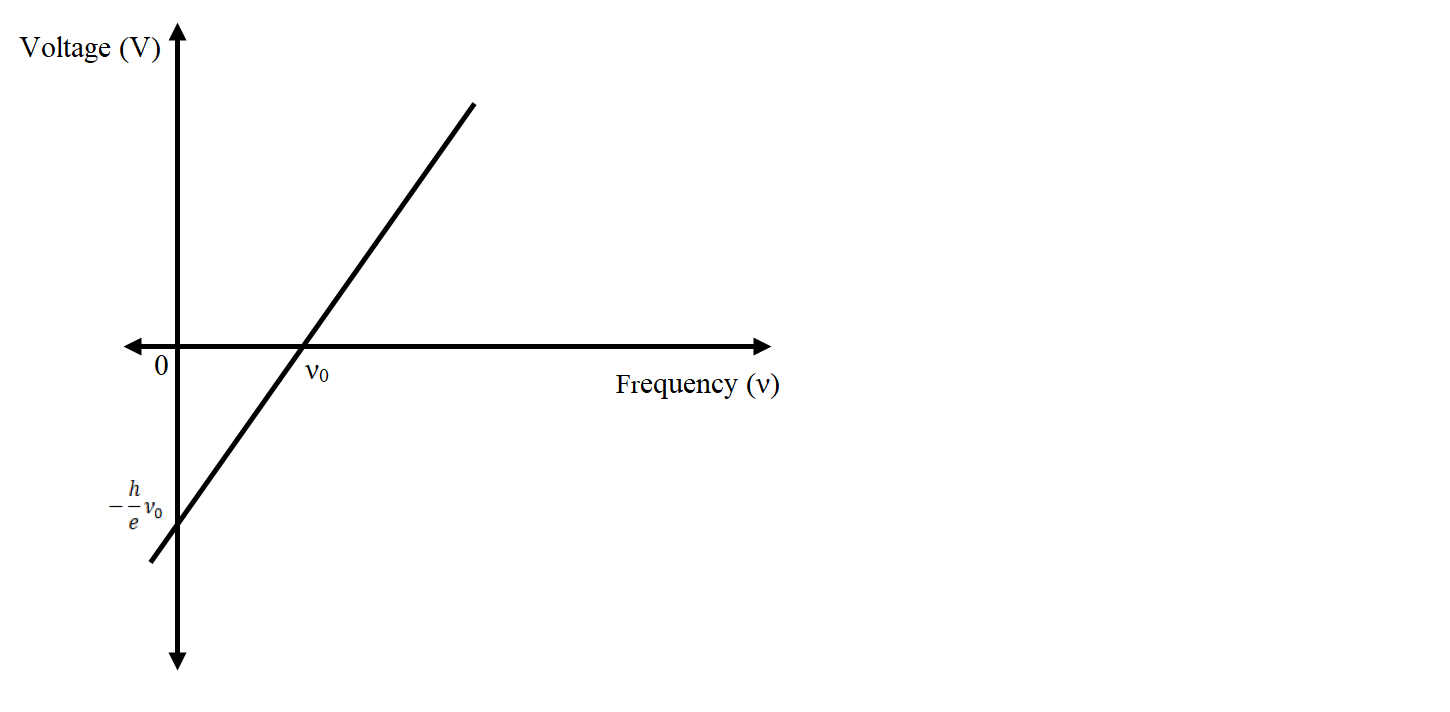
Thus, if we plot Vvs νthen we will get a straight line with slope eh and y intercept−ehνo.
The y intercept will give the cut off voltage and x intercept will give the threshold frequency.
Note: The photoelectric effect is not observed for every element. The work function and threshold frequency depend on the type of material. For a given metal if we increase frequency then the kinetic energy of photoelectrons increases and if we increase intensity then the number of photoelectrons will increase. The photoelectric effect shows the particle nature of light.
