Question
Question: Define redox reaction. Give one example....
Define redox reaction. Give one example.
Solution
Oxidation is the loss of electrons and reduction is the gain of electrons that is, a transfer of electrons takes place. The species that lose electrons are oxidized, known as reducing agents and the species that gain electrons are said to be reduced, known as oxidizing agents.
Complete step by step answer:
We now know what oxidation, reducing agent, reduction and oxidizing agent.
Let us now understand what redox reaction is. A redox reaction is when both oxidation and reduction takes place.
Redox Reaction = Oxidation + reduction
-A redox reaction can be split into oxidation half reaction and reduction half reaction.
We know what an oxidation reaction is,
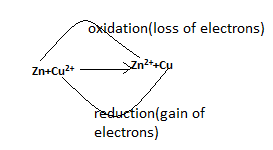
Zn→Zn2 + + 2e -
Here, the oxidation number(charge possessed by an atom) on Zn is Zero ( because in free state, the charge is zero)
-The charge on Zn2 + is 2. Thus, we can see a loss of electrons and formation of a cation. So, Zn is oxidized to Zn2 + . So, we can understand that Zn is a reducing agent
-Similarly, we can see how a reduction reaction is,
Cu2 + + 2e - →Cu
-Here, we can see that Cu2 + has a charge of 2, whereas Cu has a charge of zero.
-So, there is an addition of electrons and thus, the reaction is called as reduction reaction and Cu2 + is the oxidizing agent.
-When oxidation and reduction takes place in a chemical reaction, it is called a redox reaction. Here, one acts as a reducing agent and other as an oxidizing agent.
-Example :- Zn + Cu2 + →Zn2 + + Cu
So, in the last, redox reaction is a transfer of electrons from one species to another species.
Note:
Sometimes, when electrochemical series could not define the redox reaction properly, then it can be defined in terms of electrode potential value. When there is negative value of cell potential, it indicates a reducing agent, whereas the positive value of cell potential shows an oxidizing agent. The combination of both the reactions on electrodes indicates a redox reaction.
