Question
Question: Define peptization....
Define peptization.
Solution
It is one of the preparations of colloidal sols. It is used in the preparation of lyophobic sols because lyophobic sols cannot be prepared directly. Electrolytes are used in this process.
Complete step by step answer:
Peptization is the process of formation of colloidal sol in which conversion of fresh precipitate into colloidal particles by shaking it with the dispersion medium with the help of a small amount of suitable electrolyte. The electrolyte which is added is called a peptizing agent.
Some examples of the sols that are prepared by peptization method:
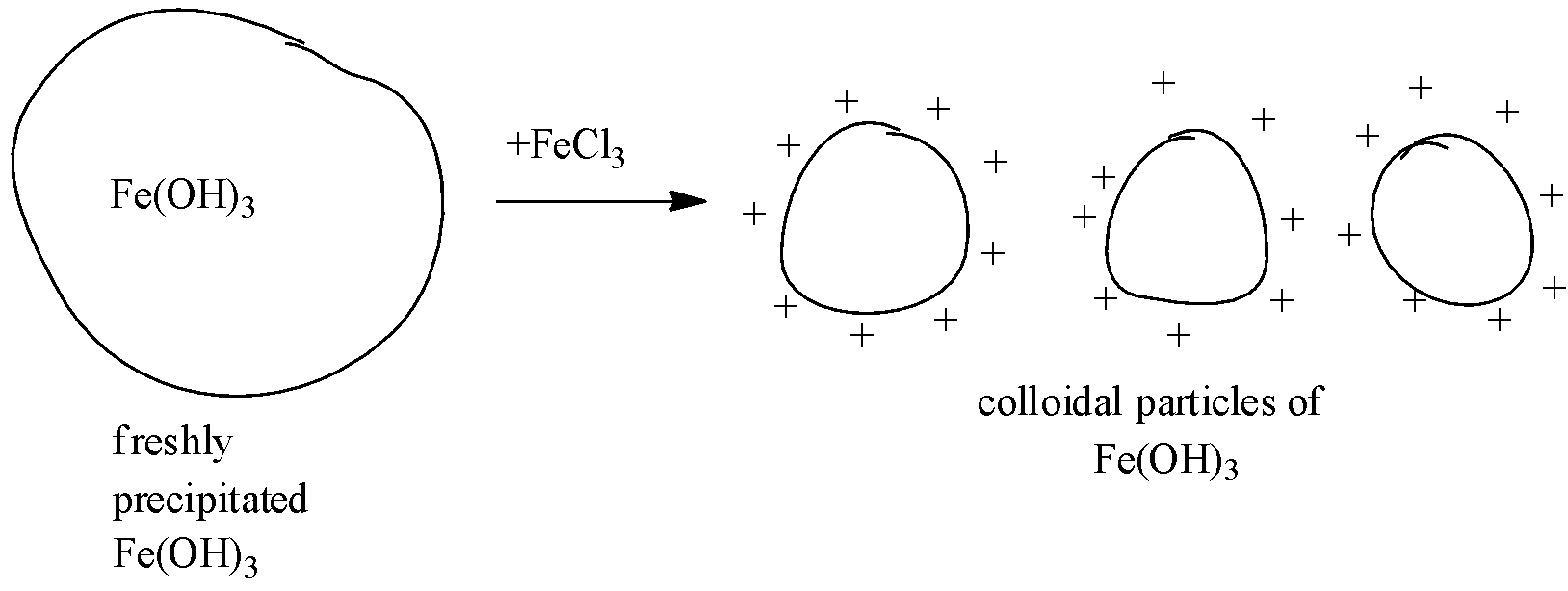
i)- A reddish-brown colored colloidal solution is obtained by adding a small quantity of ferric chloride solution to the freshly precipitates ferric hydroxide.
ii)- A precipitate of silver iodide can be peptized by shaking with a dilute solution of silver nitrate or KI.
iii)- On adding an insufficient quantity of very dilute HCl solution to the freshly precipitated aluminium hydroxide is obtained.
The peptization occurs because as the electrolyte is added to a freshly precipitated substance, the particles of the precipitate preferentially adsorb one particular type of ions of the electrolyte (ions common with the precipitated substance). As a result, they get dispersed due to electrostatic repulsion. This gives particles of colloidal size. An example of peptization of freshly precipitated ferric hydroxide with a ferric chloride solution is given in the figure. Freshly prepared preci[itates are preferred as they are easily peptized.
The figure of peptization of ferric hydroxide is:
Note: The peptization process can only occur in the presence of electrolyte which has the same ion as that of a precipitated ion. Other electrolytes cannot proceed with the procedure. So don’t be confused by thinking that any electrolyte can be used for peptization.
