Question
Question: Decreasing order of stability of following alkenes is….. 
(A)i > ii > iii > iv
(B)iv > iii > ii > i
(C)iii > iv > ii > i
(Dii > iii > I > iv
Solution
Hint: Hyperconjugation decides the stability of Alkenes. Higher the Hyperconjugation, higher will be the stability of alkenes.
Complete Step-by-Step Solution:
Hyperconjugation as well as steric strains determine the stability of Alkenes. Here, we can easily find out the more stable alkenes by giving their Hyperconjugation structures. We will see hyperconjugation in all the given alkenes respectively.
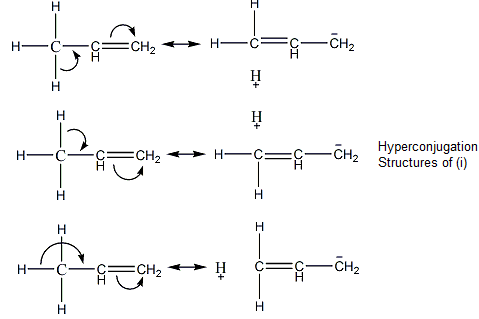
We can see that (i) alkene has 3 hyperconjugation structures.
In a similar manner, alkene (ii) will have 6 hyperconjugation structures as it has 6 α-Hydrogen atoms.
Alkene (iii) has 9 hyperconjugation structures as it has 9 α-Hydrogen atoms.
And Alkene (iv) has 12 hyperconjugation structures as it has 12 α-Hydrogen atoms.
Here, we can see that (i), (ii), (iii), (iv) have respectively 3, 6, 9, 12 hyperconjugation structures. So, we can arrange them in a way that higher the number of hyperconjugation structures, higher the stability of alkene.
So, Correct Option is (B) iv > iii > ii > i.
Additional Information:
- Hyperconjugation involves delocalization of electrons which is involved in the formation of σ bonds. So, a double bond can show hyperconjugation with H-atoms directly bonded to α-position. In short all the α-Hydrogen atoms attached with a double bond will give hyperconjugation structures.
- Hyperconjugation is also referred as “Baker-Nathan Effect” or “No Bond Resonance”.
- We can conclude that the more substituted the alkene, the more hyperconjugation structures it will have and more stable it will be.
Note:- One should know that only α-Hydrogen atoms participate in the hyperconjugation of above given alkenes. β-Hydrogen atoms need not to be counted for hyperconjugation structures.
-In these types of alkenes which have less number of C-atoms, Stearic factors do not have a major effect on stability of them.
