Question
Question: Decreasing order of acidic strength order of following compound is I > II > III ...
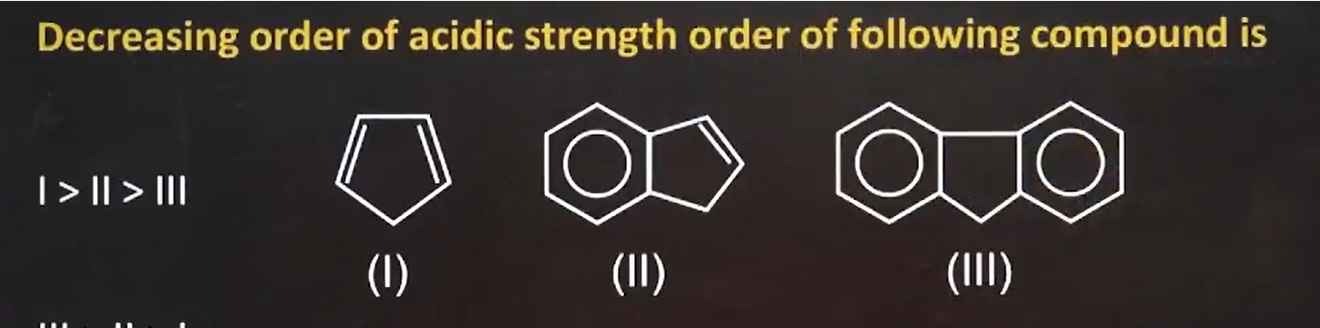
Decreasing order of acidic strength order of following compound is
I > II > III
Answer
I > II > III
Explanation
Solution
- Cyclopentadiene (I):
It is acidic (pKa ≈ 16) because deprotonation produces the aromatic cyclopentadienyl anion.
- Bicyclic compound (II):
It doesn’t form an aromatic anion upon deprotonation as effectively as (I), so it is less acidic.
- Tricyclic compound (III):
Its structure does not favor aromatic stabilization upon deprotonation and is the least acidic.
Thus, the decreasing order of acid strength is:
I>II>IIIMinimal Explanation:
Cyclopentadiene (I) is most acidic due to aromatic stabilization of its anion. Compound (II) is less acidic and compound (III) is the least acidic.
