Question
Question: Decreasing order of (-I) effect $^+NF_3 > ^+NR_3 > ^+SR_3 > ^+NH_3 > -NO_2 > -SO_3H > -CN > -CHO > ...
Decreasing order of (-I) effect
+NF3>+NR3>+SR3>+NH3>−NO2>−SO3H>−CN>−CHO>−COR>−COX>COOCOR >−COOR>−COOH>CONH2>−F>−Cl>−Br>−I>−OR>−OH>−C≡CH> −NH2>−C6H5>−CH=CH2>H
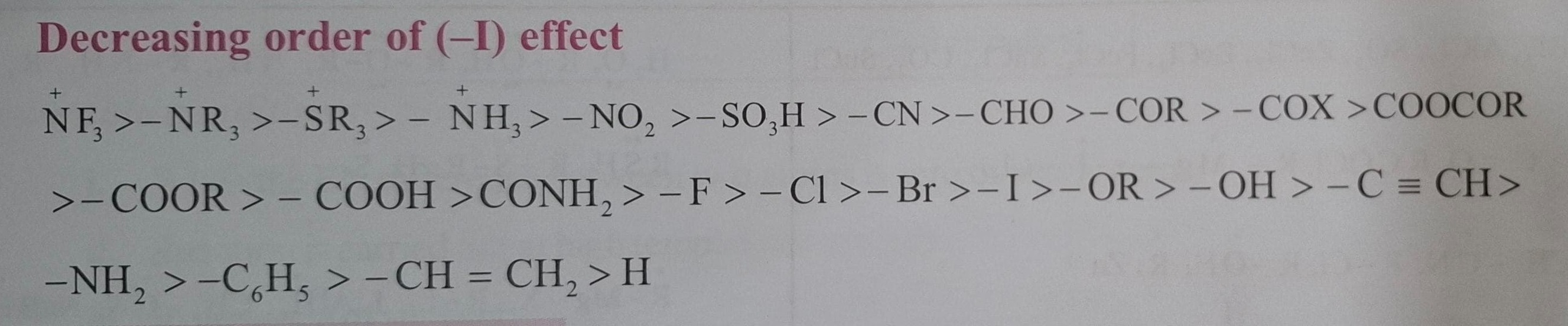
+NF3>+NR3>+SR3>+NH3>−NO2>−SO3H>−CN>−CHO>−COR>−COX>COOCOR>−COOR>−COOH>CONH2>−F>−Cl>−Br>−I>−OR>−OH>−C≡CH>−NH2>−C6H5>−CH=CH2>H
Solution
We need to arrange various substituents in order of decreasing (i.e. “most to least”) –I (inductive electron‐withdrawing) effect. One acceptable answer is as follows:
+NF3>+NR3>+SR3>+NH3>−NO2>−SO3H>−CN>−CHO>−COR>−COX>COOCOR>−COOR>−COOH>CONH2>−F>−Cl>−Br>−I>−OR>−OH>−C≡CH>−NH2>−C6H5>−CH=CH2>HMinimal Explanation
-
Key Idea: The groups with a formal positive charge (e.g. +NF3,+NR3) are very strongly electron‐withdrawing via the inductive effect.
-
Next: Classic –I groups like −NO2, −SO3H, −CN follow.
-
Then: Other carbonyl‐containing groups and halogens follow in the order of their –I strength.
-
Least: Groups like −CH=CH2 and H have little or no –I effect.
Thus, the above order is the decreasing order of the –I effect.
