Question
Question: Dative bond is present in the molecule: (A) \(N{H_3}\) (B) \(C{O_2}\) (C) \(NaCl\) (D) None ...
Dative bond is present in the molecule:
(A) NH3
(B) CO2
(C) NaCl
(D) None of there
Solution
Dative bond is a coordinate covalent bond in which two electrons derive from the same atom.
Example: The bonding of metal ions to ligands involves the dative bond.
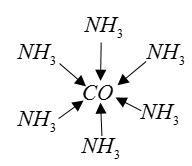
Complete Step by Step Solution:
Let us draw between the given molecules.
NH3molecule: Central atom of NH3is nitrogen; it has 5 electron forms covalent bond with their H-atoms. Two electrons remain unbonded. These are available for formation of dative bonds.
Example:
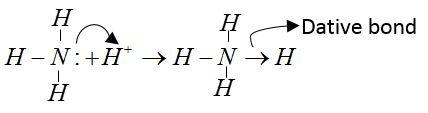
CO2 molecule: Central atom is carbon; it has 4 valence electrons. All electrons form a covalent bond with an O-atom.

C-atoms do not have lone pairs of electrons for formation of dative bonds.
NaCl molecule: NaCl is ionic molecule. It forms an ionic bond.
As follows.
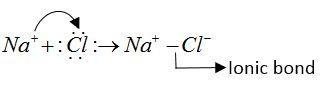
Na and Cl both atoms do not have lone pairs of electrons that form formation of dative bond.
Therefore, form the above explanation the correct option is (A) NH3.
Additional Information:
Dative bond is a chemical bond between two atoms in which only one atom provides a shared pair of electrons for bond formation.
Only those atoms form dative bonds, that have lone pairs of electrons.
Dative bond is represented by an arrowhead that points from donor atom to acceptor.
Reaction between Boron Trifluoride (BF3)and ammonia (NH3)is due to formation of dative bonds.
BF3is electron deficient and ammonia is electron rich so they form dative bonds.
Note:
Sometimes it is difficult to identify dative bonds in compounds. The complex in coordination compounds is formed by dative bonds.
Example: [Pt(NH3)3Br]+Cl−complex ion is [Pt(NH3)3Br]+dative bond present between metal Pt and ligands NH3and Br.
