Question
Question: Dative bond is present in the molecule: A) \[{\text{N}}{{\text{H}}_{\text{3}}}\] B) \[{\text{C}...
Dative bond is present in the molecule:
A) NH3
B) CO2
C) NaCl
D) PCl5
E) None
Solution
A dative bond is also known as a coordinate covalent bond. Here, both the electrons forming the bond are contributed by only one atom. Thus, it involves a one-sided sharing of a lone pair of electrons.
Complete step by step answer:
A bond formed by sharing an electron pair between two atoms is known as a covalent bond.
A bond formed by the electrostatic force of attraction between two oppositely charged ions is known as an ionic bond.
A dative bond is a special type of covalent bond where, both the electrons forming the bond are transferred by only one atom.
The structure of NH3 is as follows:
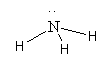
Here, all N-H bonds are formed by sharing an electron pair so all these bonds are covalent bonds.
So, option (A) NH3 is the incorrect answer.
The structure of CO2 is as follows:
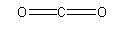
Here, both C=O bonds are formed by sharing an electron pair so both these bonds are covalent bonds.
So, option (B) CO2 is the incorrect answer.
NaCl is an ionic compound as it contains a bond between sodium metal and non-metal chlorine. So it shows ionic bonding. Ionic compounds do not show a dative bond.
So, option (C) NaCl is the incorrect answer
The structure of PCl5 is as follows:
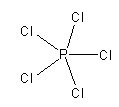
Here, phosphorus shows an exception of the octet rule as the central P atom has 10 electrons around it. As all 5 P-Cl bonds are formed by sharing an electron pair so all these bonds are covalent bonds.
So, option (D) PCl5 is the incorrect answer.
Thus, the correct option is an option (D) None.
Note: In a dative bond one atom shares its pair of electrons so it is known as a donor. While another atom accepts a pair of electrons from another atom so known as acceptor. The main difference between the covalent bond and dative bond is in covalent bond both the combining atoms share a pair of electrons while in dative bond only one atom shares a pair of electrons.
