Question
Question: Phthalimide + KOH$_{(alc)}$ → [A] [A]+ CH$_3$CH$_2$CH$_2$ Br$\overset{\triangle}{\rightarrow}$[B] [...
Phthalimide + KOH(alc) → [A]
[A]+ CH3CH2CH2 Br→△[B] [B]+H2O, OH→△[C] [D]
The final products [C] and [D] in the sequence of the above reactions are.
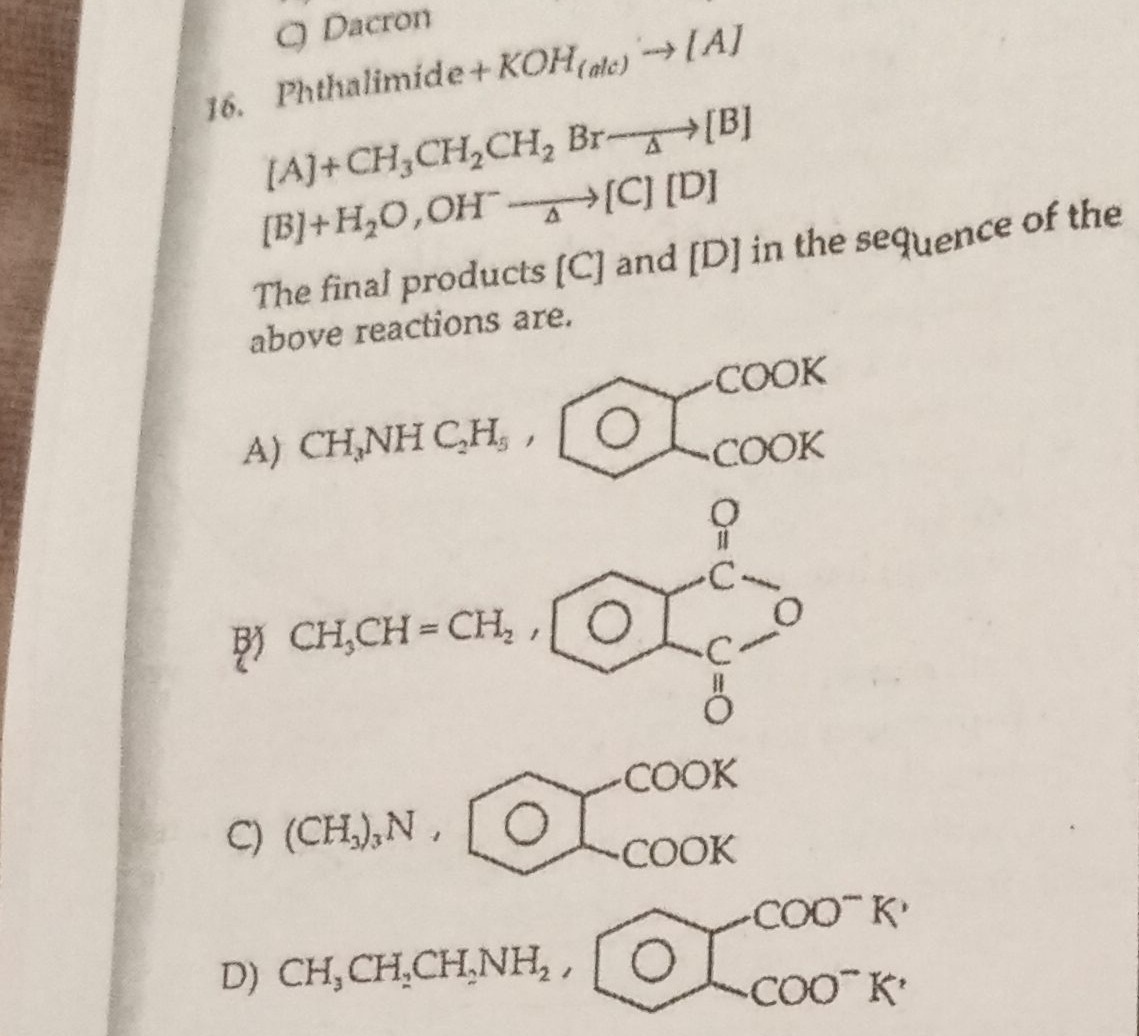
CH3NH C2H5 ,
CH3CH = CH2,
(CH3)3N ,
CH3CH2CH2NH2,
CH3CH2CH2NH2, and a benzene ring is shown with two carboxylate groups (–COO⁻K⁺) attached to adjacent carbon atoms on the ring.
Solution
The reaction sequence involves the Gabriel synthesis.
-
Formation of [A]: Phthalimide reacts with alcoholic KOH to form potassium phthalimide.
-
Formation of [B]: Potassium phthalimide reacts with CH3CH2CH2Br (an alkyl halide) to yield N-propylphthalimide.
-
Formation of [C] and [D]: Hydrolysis of N-propylphthalimide yields n-propylamine ([C], CH3CH2CH2NH2) and potassium phthalate ([D], a benzene ring with two -COO−K+ groups at adjacent positions).
