Question
Question: In the following sequence of reactions: $CH_3CH_2OH \xrightarrow{KMnO_4} (X) \xrightarrow{SOCl_2/NH...
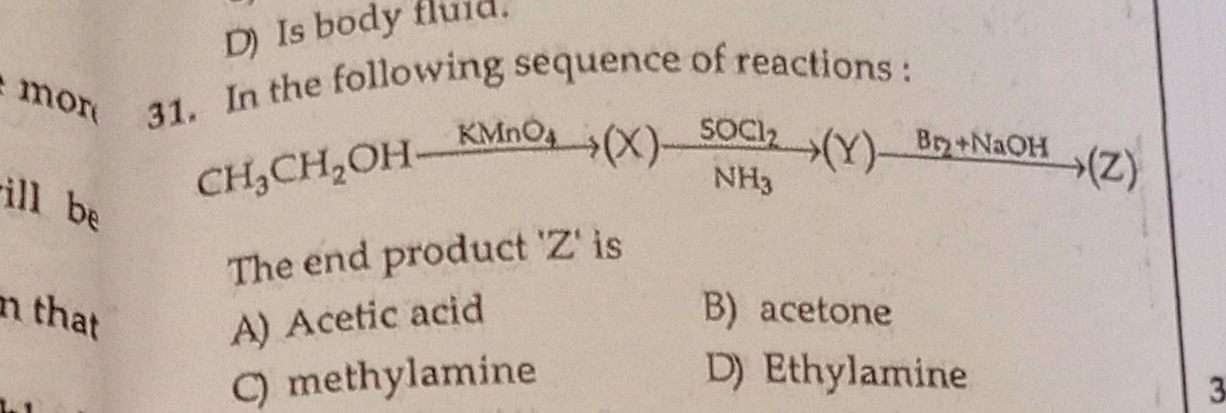
In the following sequence of reactions:
CH3CH2OHKMnO4(X)SOCl2/NH3(Y)Br2+NaOH(Z)
The end product 'Z' is
A
Acetic acid
B
acetone
C
methylamine
D
Ethylamine
Answer
methylamine
Explanation
Solution
The reaction proceeds as follows:
-
Oxidation: Ethanol (CH3CH2OH) is oxidized by KMnO4 to acetic acid (CH3COOH).
CH3CH2OHKMnO4CH3COOH
-
Conversion to Amide: Acetic acid is converted to acetyl chloride (CH3COCl) using SOCl2, which then reacts with ammonia (NH3) to form acetamide (CH3CONH2).
CH3COOHSOCl2CH3COCl
CH3COCl+NH3→CH3CONH2
-
Hofmann Rearrangement: Acetamide undergoes Hofmann rearrangement in the presence of Br2 and NaOH to yield methylamine (CH3NH2).
CH3CONH2Br2+NaOHCH3NH2+CO2
Therefore, the end product 'Z' is methylamine.
