Question
Question: D(+)-glucose reacts with hydroxyl amine and yields oxime. What would be the structure of the oxime?...
D(+)-glucose reacts with hydroxyl amine and yields oxime. What would be the structure of the oxime?
Solution
The molecular formula of hydroxyl amine isNH2OH. Generally hydroxyl amine reacts with carbonyl carbon and forms respective oxime as a product. Glucose also reacts with hydroxyl amine due to the presence of the aldehyde functional group in D(+)-glucose.
Complete step by step answer:
-The structure of D(+)-glucose is as follows.
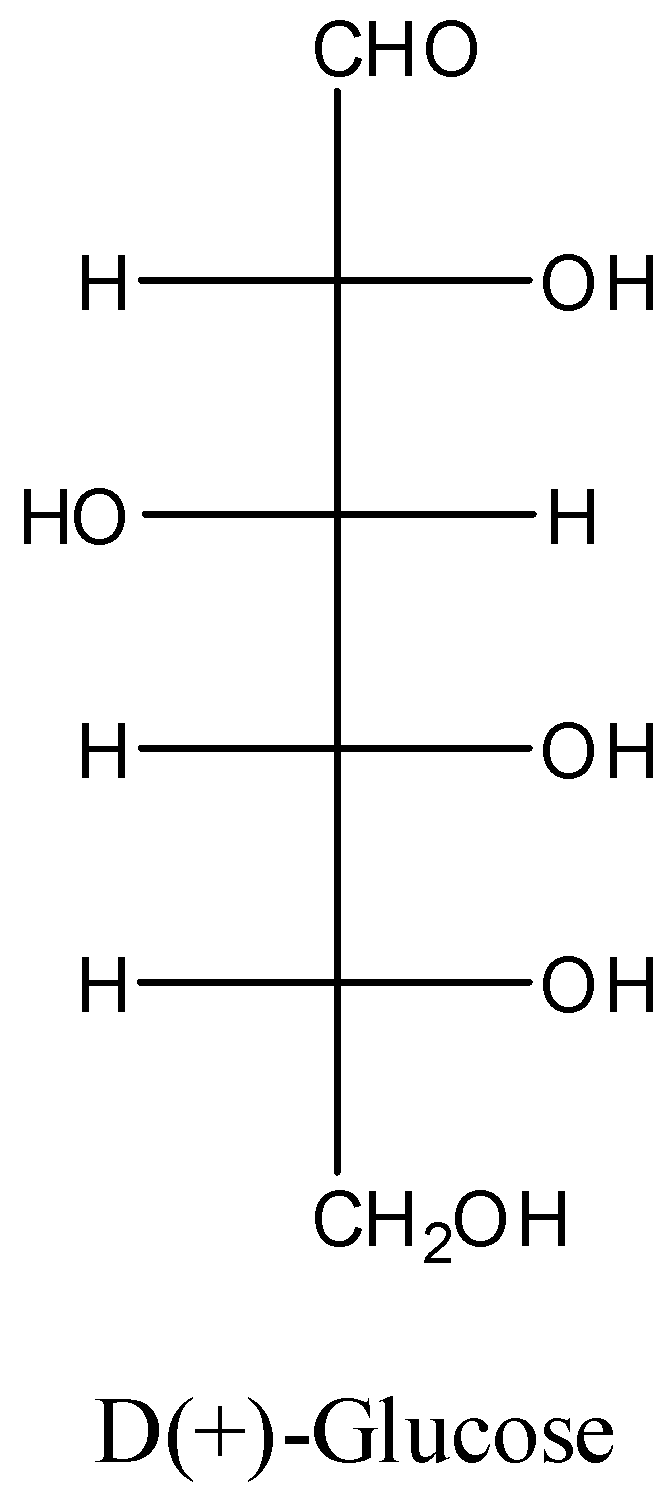
-The D(+)-glucose contains aldehyde groups in its structure.
-Then the aldehyde reacts with hydroxylamine and forms aldoxime structure.
-The chemical reaction of D(+)-glucose with hydroxylamine is as follows.
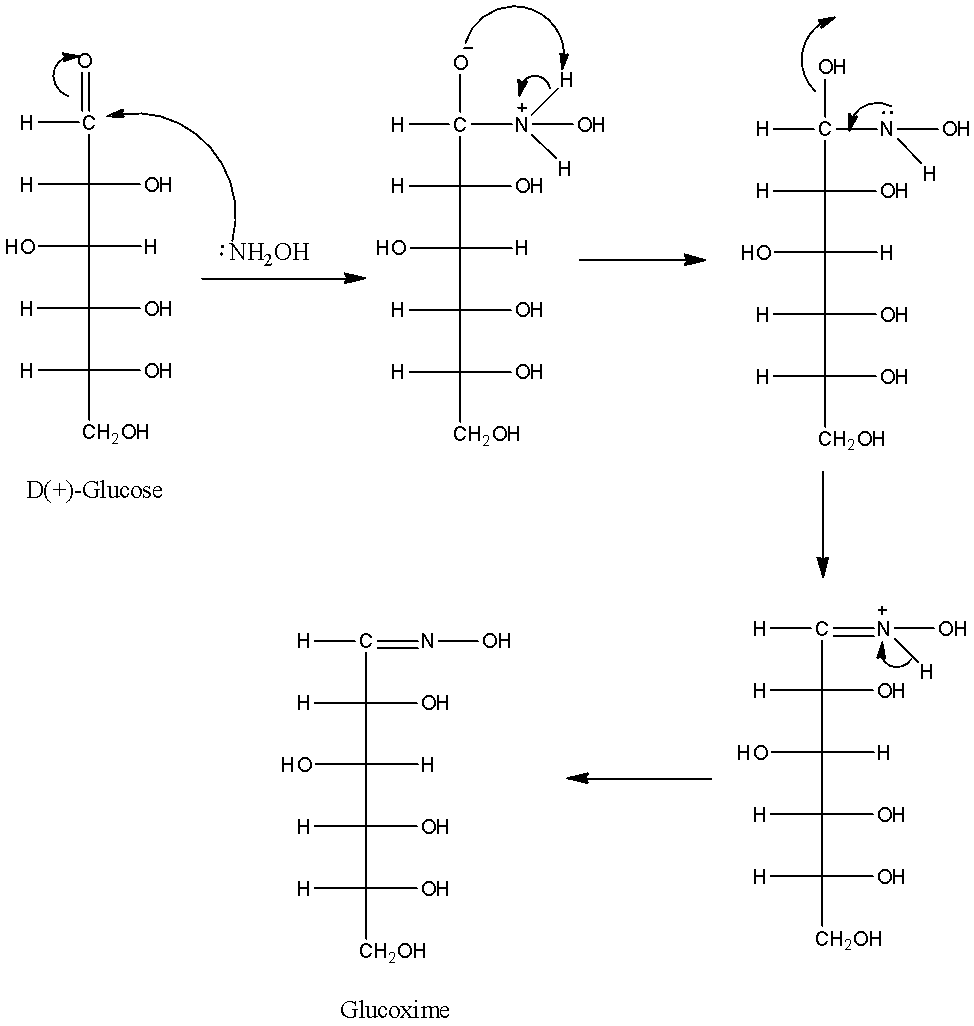
-The structure of the glucoxime is as follows.
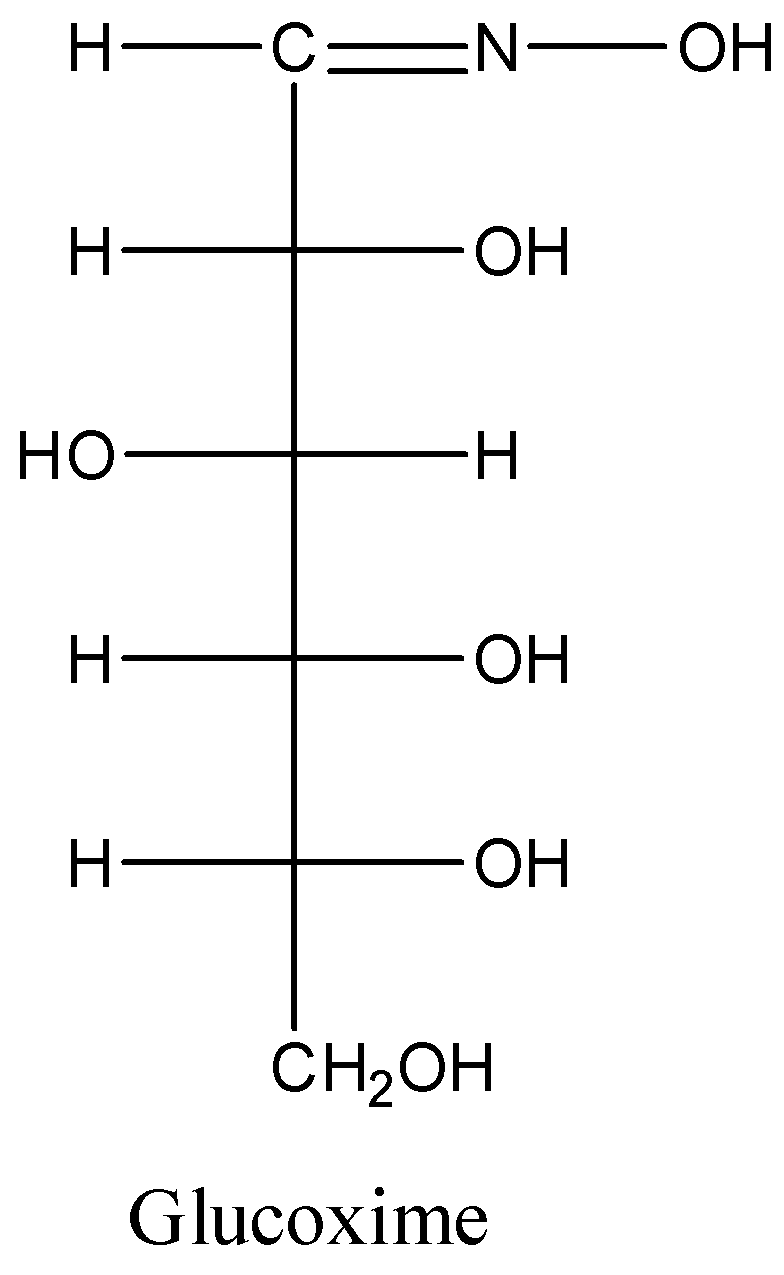
Additional information:
-Almost maximum oximes are solids in nature and it is useful to find the presence of aldehyde or ketone functional groups in the given compound.
-Oximes are colorless crystals and less soluble in water.
-Oxime derivatives are used as medicines for nerve agents (A nerve agent deactivates acetylcholinesterase by phosphorylation reaction).
-Oximes are the chemical derivatives and belong to the category of imines.
-Dimethylglyoxime is a famous oxime used in the analysis of nickel.
-Occasionally oximes involves in multistep chemical synthesis to guard carbonyl compound
-Oximes exhibit both acidic (Weak acid) and base properties and are toxic in nature.
-Oximes are going to decompose while heating and create massive explosions.
Note: In Japan, perillaldehyde (an oxime) is used as an artificial sweetener. Methyl ethyl ketoxime is used in oil paints and as a preservative for avoiding the skin from chemicals. Acetone oxime is used to reduce the corrosion which reduces the toxicity.
